To study biomechanical forces in multicellular systems, effective mechanical stimulation is an essential tool.
A 3D microstructure device is developed using Two-Photon Polymerization (2PP)-based 3D printing for mimicking the human multicellular environment. Utilizing Nanoscribe's IP-PDMS and BIO INX bioresins, the device enables high-fidelity 3D organotypic cell cultures. The encapsulation of multiple cell types and precise mechanical stimulation via a cantilever trigger specific cellular responses and morphological changes in the device. This innovative approach allows detailed studies of mechanical stimuli on multicellular systems. It has various potential applications in stem cell-based organoid cultures, cancer cell spheroids, and mechanical force studies in knee joints to understand osteoarthritis and rheumatoid arthritis. The device’s versatility highlights its significant potential in biomedical research.
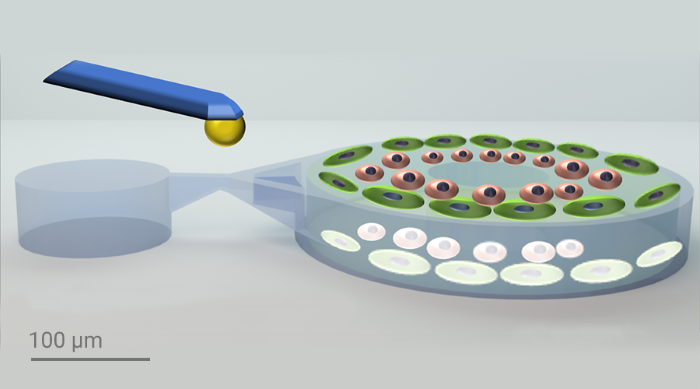
Image Credit: Nanoscribe
Cellular activities such as development, morphogenesis, and metabolism are significantly influenced by mechanical forces. While previous research has examined cellular responses to mechanical stimuli in two-dimensional environments, replicating these complex conditions in a controlled, three-dimensional setting has been challenging. Researchers at the University of Heidelberg, part of the Cluster of Excellence 3D Matter Made to Order (3DMM2O) have tackled this issue using Nanoscribe‘s 3D Microfabrication technology to create high-resolution, multifunctional 3D microstructures. They developed a device with an outer layer of IP-PDMS and an inner part made of HYDROBIO INX N400 bioresin mixed with living cells, enabling precise mechanical stimulation and the study of multicellular systems under defined mechanical forces.
Developing 3D microdevices for biomechanical studies
The researchers from the Selhuber-Unkel lab designed the device using CAD software and optimized it with finite element analysis to predict strain under mechanical stimulation. The outer part of the device was printed using IP-PDMS and the inner part using Hydrobio INX N400, which is optimized for printing with living cells. The researchers verified the print quality using scanning electron microscopy (SEM), brightfield and fluorescence microscopy, ensuring minimal swelling and shrinkage. The final devices were capable of supporting multicellular systems and enabling precise mechanical stimulation using a cantilever.
Superior choices for 3D multimaterial printing
Multi-material printing was used to create a 3D microstructured device that mimics complex mechanical environments in vivo. Nanoscribe’s IP-PDMS was selected for its elasticity and flexibility, making it ideal for cell scaffolding and tissue engineering. As the softest resin that Nanoscribe offers, with a Young’s modulus of 15.3 MPa, IP-PDMS enabled durable, flexible structures. HYDROBIO INX N400, a natural gelatin-based hydrogel was selected for its compatibility with live-cell printing. Human fibroblast-like synoviocytes (HFLS), as a model for studying inflammatory joint diseases, and human umbilical vein endothelial cells (HUVEC), selected for their location in joint linings, were encapsulated in N400 to create a realistic multicellular environment.
Investigating morphological changes in stimulated cells
The printed 3D microstructure device underwent several rigorous tests to verify its functionality and effectiveness. For the mechanical testing, a nanoindenter equipped with a cantilever of known stiffness was used to apply controlled forces on the device, specifically targeting the IP-PDMS bridge to induce precise displacement within the hydrogel cylinder containing the cells. The forces applied ranged from 200 μN at frequencies of 0.5 Hz to 1 Hz. At 1 Hz, the higher frequency resulted in less displacement due to the hydrogel-based resin properties, leading to fewer morphological changes in the cells. When subjected to mechanical stimulation at 0.5 Hz for 30 minutes, the cells exhibited significant morphological changes and actin remodeling, with actin fibers showing reorganization and alignment in response to the cyclic mechanical stretch. As a proof of concept, Medaka retinal organoids were encapsulated within the device to demonstrate that preformed organoids can also be effectively stimulated using this method
Project team
- Institute for Molecular Systems Engineering and Advanced Materials (IMSEAM) – Heidelberg University
- Centre for Organismal Studies – Heidelberg University
- Cluster of Excellence – 3D Matter Made to Order (3DMM2O)
Read the publication here
- Two-Photon Laser Printing to Mechanically Stimulate Multicellular Systems in 3D
Discover Quantum X bio
- High-performance 3D bioprinter with nanoprecision
Improving efficiency and quality in 3D bioprinting
The 3DMM2O scientists attained impressive outcomes despite the stitching necessary to print their device with the Nanoscribe Photonic Professional GT2. A possibility to eliminate stitching and gain additional advantages would be to use a highest-precision 3D bioprinter, Nanoscribe’s Quantum X bio. This printer offers a larger print field, allowing for stitching-free prints of the 3D microstructure device that was printed for this work. The advanced alignment function of the Quantum X bio would facilitate the secondary printing process with HYDROBIO INX N400, reducing potential errors by ensuring precise superposition. Furthermore, the Quantum X bio provides controlled temperature and humidity environments, HEPA-filtered airflow, and optional connections for pre-mixed air/CO2, leading to more reliable and higher quality results. As the go-to printer for live-cell printing, the Quantum X bio supports a wide range of bioprinting applications, accommodating diverse setups such as sterile cell culture dishes, microscope slides, and microfluidic chips.
The researchers conclude that their 3D microprinted device can be easily customized to investigate different cell types, such as cardiac cells, and modify the shape and thickness of the printed device, the substrate it is printed on, or the resins used, including conductive ones. The Quantum X bio's openness to various materials would further support these customization options, enabling researchers to tailor their experiments more precisely. This capability could enhance experimental conditions and provide more versatile research opportunities, potentially leading to significant advancements in their field.
Video Credit: Nanoscribe