The primary carnitine shuttle system in mitochondria comprises two enzymes (carnitine palmitoyl transferase 1 [CPT1] and CPT2) and an inner mitochondrial membrane transporter, carnitine/acylcarnitine carrier (CAC), also known as solute carrier 25 member 20 (SLC25A20). CAC is crucial for transporting acylcarnitines into mitochondria for β-oxidation. Notably, no redundant mitochondrial transporter compensates for CAC deficiency, making it indispensable for cellular life. Other carnitine shuttles are active in the endoplasmic reticulum and peroxisomes.
The coordination of peroxisomal and mitochondrial carnitine shuttles is essential for fatty acid catabolism. Carnitine has been implicated in modulating the acetyl-coenzyme A (CoA)-to-CoA ratio, which profoundly impacts lipid biosynthesis, gene expression, and carbohydrate metabolism. In the present study, researchers reviewed the role of transporters in carnitine traffic, focusing on the relationship between carnitine and fertility.
Carnitine network and alterations
Carnitine distribution is highly variable across tissues, ranging from low millimolar levels in most tissues to the highest (60 mM) in the testes. Many transporters are involved in maintaining carnitine homeostasis. Diet plays a crucial role in carnitine distribution; in fish and meat consumers, dietary carnitine accounts for about 75% of the total carnitine content. In contrast, vegans and vegetarians often rely heavily on endogenous synthesis and renal reabsorption for maintaining carnitine levels. Without supplements, vegans and vegetarians may experience reduced carnitine levels.
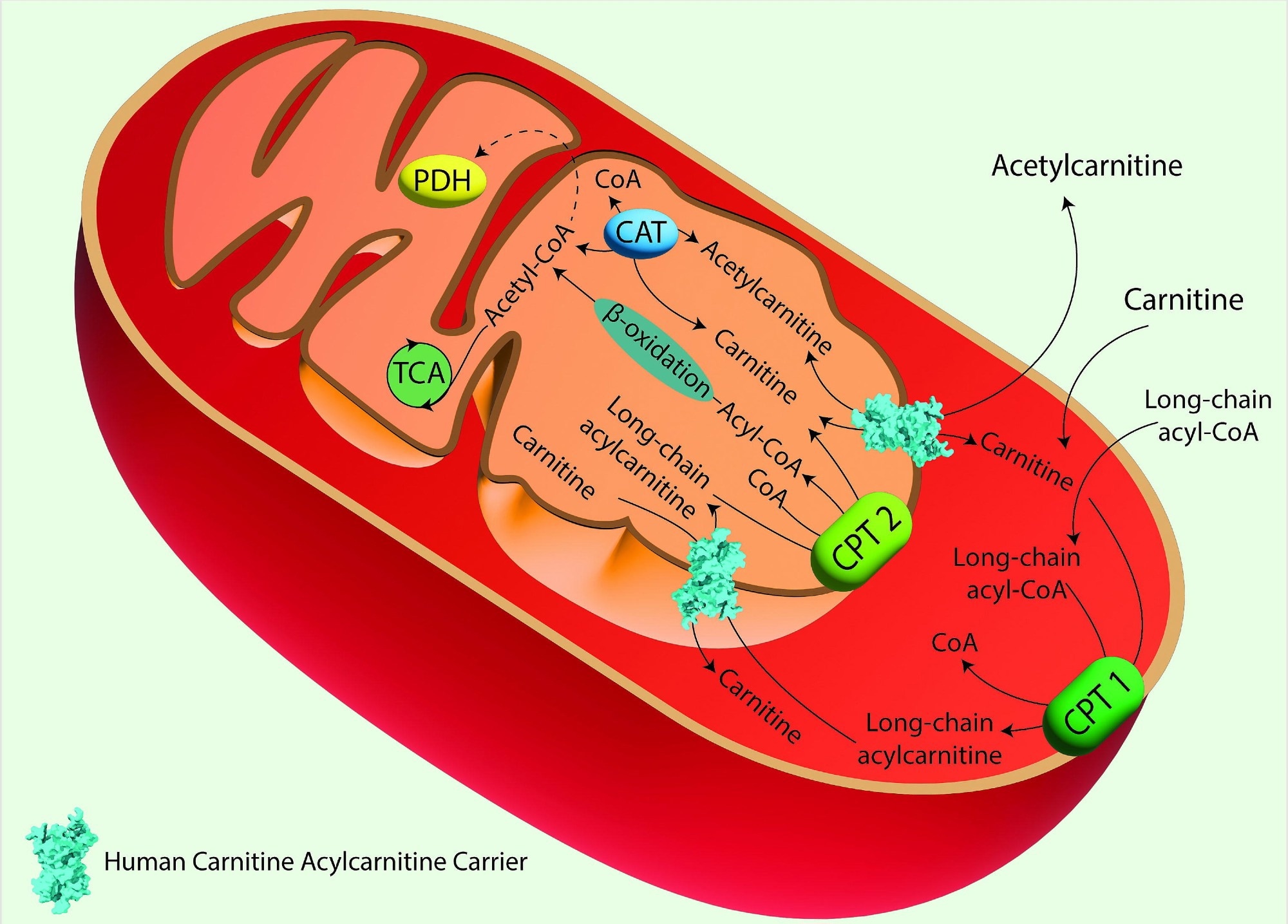 The Carnitine Shuttle in Mitochondrial Fatty Acid Oxidation. Acyl-CoA synthase catalyzes the conversion of long-chain fatty acids into fatty acyl-CoAs. These are then converted to acylcarnitines by Carnitine Palmitoyl Transferase 1 (CPT 1), which is located in the outer mitochondrial membrane. Acylcarnitines are transported across the inner mitochondrial membrane by the Carnitine/Acylcarnitine Carrier (CAC) in exchange for free carnitine. Once inside the mitochondrial matrix, Carnitine Palmitoyl Transferase 2 (CPT 2), located on the inner mitochondrial membrane, converts acylcarnitines back into acyl-CoAs and free carnitine. The free carnitine is transported to the cytosol by the CAC, and it can be recycled by CPT 1. The acyl-CoAs imported into the mitochondrial matrix through the carnitine shuttle are subjected to β-oxidation, producing acetyl-CoA, which can then enter the TCA. Image created using Adobe Illustrator. hCAC is represented as space fill model from AlphaFold prediction
The Carnitine Shuttle in Mitochondrial Fatty Acid Oxidation. Acyl-CoA synthase catalyzes the conversion of long-chain fatty acids into fatty acyl-CoAs. These are then converted to acylcarnitines by Carnitine Palmitoyl Transferase 1 (CPT 1), which is located in the outer mitochondrial membrane. Acylcarnitines are transported across the inner mitochondrial membrane by the Carnitine/Acylcarnitine Carrier (CAC) in exchange for free carnitine. Once inside the mitochondrial matrix, Carnitine Palmitoyl Transferase 2 (CPT 2), located on the inner mitochondrial membrane, converts acylcarnitines back into acyl-CoAs and free carnitine. The free carnitine is transported to the cytosol by the CAC, and it can be recycled by CPT 1. The acyl-CoAs imported into the mitochondrial matrix through the carnitine shuttle are subjected to β-oxidation, producing acetyl-CoA, which can then enter the TCA. Image created using Adobe Illustrator. hCAC is represented as space fill model from AlphaFold prediction
As such, endogenous synthesis and reabsorption may be more relevant for homeostasis. Renal reabsorption of carnitine is the primary means of compensating for dietary carnitine deficiencies. Organic cation/carnitine transporter 2 (OCTN2) facilitates carnitine reabsorption in the kidneys; mutations in OCTN2 lead to primary carnitine deficiency (PCD), a disorder characterized by systemic carnitine depletion and associated clinical manifestations, including muscle weakness, cardiomyopathy, and infertility. PCD treatment involves lifelong carnitine supplementation.
Secondary carnitine deficiencies (SCDs) may occur due to inherited defects in CPT2, CAC, or acyl-CoA dehydrogenases. Notably, CAC defects often result in life-threatening conditions due to impaired mitochondrial β-oxidation, leading to increased fatty acid accumulation in the cytoplasm. There are no redundant carnitine transporters to compensate for CAC defects. Therefore, early intervention is paramount for CAC deficiency. Treatment strategies for SCDs include diet-induced hypoglycemia prevention, carnitine supplementation, and medium-chain fatty acid diets.
Carnitine and Oxidative Stress in Infertility
Oxidative stress plays a pivotal role in both male and female infertility, with reactive oxygen species (ROS) and reactive nitrogen species (RNS) contributing to lipid peroxidation, DNA fragmentation, and reduced sperm viability. Carnitine, with its antioxidant properties, helps mitigate oxidative damage by scavenging free radicals, protecting sperm mitochondria from oxidative stress, which is particularly important in the preservation of sperm motility and overall fertility. The study also highlights how the antioxidant function of carnitine is crucial in managing oxidative stress in female reproductive tissues, such as the ovaries, where ROS imbalances can impair oocyte quality and disrupt the endometrial environment.
Human infertility
Globally, infertility is a significant concern, affecting over 180 million couples. Historically, it was predominately attributed to females; however, contemporary insights highlight a significant male contribution. The cause of infertility remains unexplained in many cases despite diagnostic advances. Mitochondrial dysfunctions are emerging as a common factor in both male and female infertility, linking energy metabolism to reproductive health. Lifestyle choices influence fertility, with evidence linking alcohol, obesity, and smoking to poor semen quality and ovulatory function.
Further, despite multiple studies on the relationship between male fertility and carnitine, the underlying molecular mechanisms remain elusive. In the male reproductive system, carnitine facilitates mitochondrial energy metabolism, particularly by regulating the acetylcarnitine/CoA ratio, which putatively is responsible for sperm concentration and motility. This regulation is particularly important in sperm maturation, where carnitine concentrations increase dramatically from the epididymal head (5 mM) to the tail (60 mM).
Some studies have suggested that lumen carnitine may stabilize the plasma membrane of sperms, enhance survival, and mitigate acrosome-reacted sperms, which are critical for successful fertilization.
The role of carnitine in females is less clear. However, carnitine is involved in the energy supply necessary for ovulation, folliculogenesis, and embryonic development. Mitochondrial dysfunction, exacerbated by carnitine deficiency, has been implicated in conditions like polycystic ovary syndrome (PCOS) and endometriosis. Therefore, carnitine deficiency could result in suboptimal energy, compromising oocyte quality and reducing the fertilization potential. Furthermore, carnitine has been shown to influence the production of sex hormones such as testosterone, estrogen, and progesterone, which are crucial for reproductive health.
SLCs in the carnitine network
Studies indicate that OCTN1 might function as a carnitine transporter in tissues with high carnitine levels, such as the epididymis. However, direct links between OCTN1 and infertility have not been studied so far. OCTN2 is the highest-affinity carnitine transporter. It is ubiquitously expressed in the heart, skeletal muscle, kidneys, and intestine. Mutations in OCTN2 are associated with Crohn’s disease (CD) due to carnitine deficiency in the intestinal epithelium. Further, mutations in OCTN2 can lead to fertility issues by disrupting carnitine homeostasis in the reproductive system, particularly in the epididymis. In the male reproductive system, carnitine transporter 2 (CT2) is localized in the luminal membrane of epididymal cells and the plasma membrane of Sertoli cells in the testes. In females, CT2 is highly expressed in the endometrium.
CT2 localization in the testes is the primary molecular link between male infertility and carnitine; however, its presence in the endometrium or testes does not clarify whether carnitine is associated with transporter regulation or energy requirements. CAC is an indispensable protein for cellular life. While the study points to CAC’s potential role in infertility, no direct causal mutations linking CAC to fertility dysfunction have been confirmed yet.
Clinical Implications
The study concludes by suggesting potential treatment avenues. Carnitine supplementation has shown promise in improving sperm motility and morphology in idiopathic infertility cases, while its role in antioxidant defense highlights its therapeutic potential in oxidative-stress-related reproductive disorders. Recognizing the role of carnitine and SLCs in sperm motility and energy metabolism could help develop advanced, more refined diagnostic tools and targeted therapies. Further research is necessary to understand the exact molecular mechanisms linking carnitine transporters and reproductive health, which could lead to novel treatments for both male and female infertility.