Background
Human cytomegalovirus (HCMV) is a virus that causes congenital infections and serious health complications, especially in immunosuppressed individuals. Transmission from mother to fetus can lead to neurodevelopmental delays, hearing loss, and other severe disabilities. Moreover, despite substantial advances in antiviral therapies, their use in treating HCMV infections is limited by factors such as toxicity and resistance, leaving vaccination as a critical but unmet need.
While natural immunity provides partial protection, it is imperfect and does not prevent reinfections. Studies have shown that pre-conception maternal immunity can reduce but not eliminate vertical transmission, with risks persisting for both primary and recurrent infections. Similarly, transplant recipients face elevated risks of HCMV reactivation, graft failure, and opportunistic infections, further emphasizing the necessity for vaccination in this population.
HCMV Vaccine Development
The review explored the ongoing efforts to develop effective HCMV vaccines, focusing on multiple vaccine platforms and trial designs. The authors analyzed the development of subunit, deoxyribonucleic acid (DNA), messenger ribonucleic acid (mRNA), viral vector, and live-attenuated HCMV vaccines.
The development of vaccines against HCMV has spanned decades, with several promising candidates undergoing clinical trials. Early research concentrated on live-attenuated vaccines, such as the Towne and Toledo strains of the virus, which provided partial protection but did not significantly reduce virus acquisition.
The study reported that subsequent subunit vaccines targeted glycoprotein B, a key viral entry protein. These vaccines demonstrated modest efficacy, with phase II trials showing approximately 50% protection in specific populations, such as seronegative individuals and transplant recipients.
Recent innovations in mRNA technology have significantly advanced vaccine development, particularly Moderna's mRNA-1647. This candidate encodes glycoprotein B and the pentameric complex—key viral structures for cell infection—and has reached phase III clinical trials. These mRNA-based vaccines aim to elicit strong neutralizing and non-neutralizing antibody responses, including mechanisms like antibody-dependent cellular cytotoxicity (ADCC). Additionally, virus-like particle (VLP) vaccines, such as those developed by VBI Vaccines Inc., also incorporate glycoprotein B and other antigens to stimulate broader immune responses.
Another approach discussed in the review involved the use of viral vectors, such as the Triplex vaccine, which leverages modified vaccinia Ankara to express HCMV proteins. This candidate vaccine showed promise in transplant recipients by reducing viremia and disease severity.
Other innovations included disabled infectious single-cycle (DISC) vaccines, such as V160, developed by Merck. These vaccines present viral antigens without replicating and are currently being tested in women of childbearing age.
The review underscored that these diverse vaccine platforms not only provide insights into the design of effective immunogens but also highlight the importance of integrating multiple immune mechanisms, including T-cell responses and innate immunity, to achieve comprehensive protection.
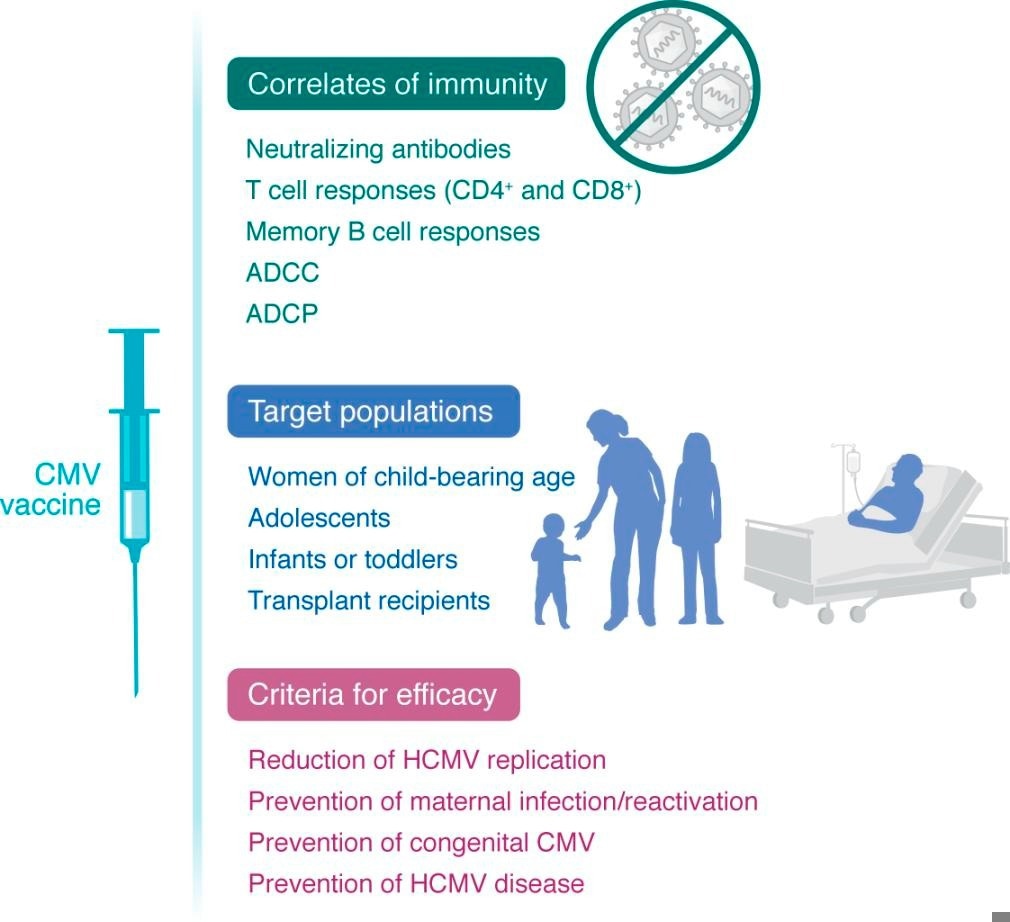
Schematic of the types of immunity, target population, and endpoints for efficacy of a CMV vaccine.
Challenges and Future Directions
Developing an effective HCMV vaccine has presented substantial hurdles, including the virus's complex biology, immune evasion mechanisms, and diverse clinical impacts. A significant challenge has been the incomplete immunity provided by natural infection, which complicates the development of vaccines that can prevent reinfections or congenital transmission.
The high variability of HCMV strains necessitates designing vaccines that target conserved viral components while addressing strain-specific differences. In addition to focusing on glycoprotein B and the pentameric complex, emerging research highlights the need to optimize immune responses, such as antibody-dependent cellular phagocytosis (ADCP).
Furthermore, the researchers believe that clinical trials must also address the complexities of demonstrating efficacy. Unlike traditional vaccines, the endpoints for HCMV must include preventing congenital infections and reducing complications in transplant recipients. Moreover, the vaccine's safety profile is especially crucial for immunocompromised populations.
The review also highlighted equity considerations, noting that congenital HCMV disproportionately affects low- and middle-income countries, where seroprevalence is higher. Developing vaccines tailored to these populations could have a transformative global health impact.
Emerging technologies, such as mRNA platforms and structure-based vaccine design, offer new opportunities to overcome these challenges. Strategies that are currently under investigation include incorporating multiple antigens, enhancing T-cell responses, and targeting viral latency. Addressing gaps in knowledge, such as the role of non-neutralizing antibodies and the impact of maternal immunity, is also essential for optimizing vaccine candidates.
Conclusions
Overall, the review suggested that the future of HCMV vaccine development lies in multifaceted approaches that integrate innovative platforms, comprehensive immune assessments, and tailored trial designs. Advancing vaccine candidates through accelerated regulatory pathways and fostering collaborations across global stakeholders are critical steps toward addressing this urgent public health need.
Journal reference:
- Permar, S. R., Schleiss, M. R., & Plotkin, S. A. (2025). A vaccine against cytomegalovirus: how close are we? The Journal of Clinical Investigation, 135, 1. DOI:10.1172/JCI182317, https://www.jci.org/articles/view/182317