Introduction
This article provides ten top tips which are important for obtaining positive ChIP results. Below are details of the steps that can be taken to achieve these results.
1. Chromatin Quality Critical for Successful ChIP Assay
In quantitative analyses, the quality of the chromatin prepared is an important factor that determines whether a ChIP assay is successful. The three main aspects of chromatin preparation are lysis, fixation and shearing, with each of these needing to be fully optimized.
2. Keep Chromatin on Ice
Chromatin rapidly degrades, particularly when it is stored at room temperature. For optimum results, chromatin must be kept on ice throughout the whole experiment, although freeze/thaw cycles should not be used.
One way to help prevent degradation is to use small aliquot quantities of chromatin after the stock has been prepared. In the case of Chromatrap® technology, very small amounts of chromatin are required per IP and small aliquot amounts are therefore preferred.
3. Optimize Cell Fixation
In order to ensure high quality chromatin, care should be taken to optimize cell fixation. This is because overly fixed cells may become resistant to shearing and lysis, thereby reducing the efficiency of cross-linking and reducing downstream signal clarity.
Chromatrap® enables cell fixation with just 1% formaldehyde in PBS or media. A rotating platform should be used for ten minutes but if this rate gives a poor yield, five minutes is usually adequate.
4. Use Fresh Fixation Solution
More reproducible results can be obtained if fresh formaldehyde is used for every chromatin preparation. The formaldehyde should not contain any methanol because methanol can affect cell membranes.
5. Choose Appropriate Cell Shearing Technique
Both enzymatic (micrococcal nuclease digestion) shearing and sonication (mechanical shearing utilizing ultrasonics) are effective shearing methods, but it is important to select the right shearing technique for the cells. Standard ChIP kits from Chromatrap® offer both shearing methods, as well as a separate enzymatic shearing kit for chromatin optimization.
For cases where a sonicator is not available, enzymatic shearing can be used because it causes less disruption to the protein epitopes detected by the specific antibody. On the other hand, this method can create bias owing to nuclease sequence specific cleavage. Furthermore, certain cell types could be resistant to lysis and sonication could therefore be helpful in both lysis and shearing. In such circumstances, sonication can prove useful in lysis and shearing.
Enzymatic shearing is necessary when performing native ChIP because sonication can disrupt the protein/DNA complexes. Table 1 shows the pros and cons of shearing optimization using enzymatic digestion and sonication.
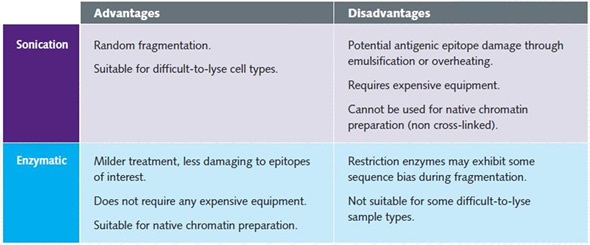
Table 1. Advantages and disadvantages of optimization of shearing through sonication and enzymatic digestion.
6. Shear Chromatin to Ideal Fragments
Every chromatin preparation must be sheared to fragments that are between 100 and 500 base pairs (bps) in length. Quantitative analysis of chromatin can be performed using a fluorometer, spectrophotometer or a microfluidics platform, while for qualitative analysis, a microfluidics platform or agarose gel is required. Chromatrap recommends that a microfluidics platform is used to perform DNA quantification as it offers the greatest precision and is also highly compatible with Chromatrap® buffer systems.
Both under shearing and over shearing of chromatin are associated with disadvantages. Under shearing will result in larger fragments and increase non-specific binding in the ChIP assay, while over shearing produces tiny fragments, which can lower primer recognition and therefore PCR. In addition, over shearing by sonication can damage the protein epitopes.
Sonication efficiency will not only differ between cell types, but can also be influenced by the degree of heating, cross linking, and emulsification of the sample. These factors will all reduce shearing efficiency. It is critical to optimize sonication in order to obtain a successful ChIP result.
Sonication tubes that are made from harder plastic transfer ultrasonic waves more efficiently than tubes made of softer plastic. First, optimize an abundant tissue sample or cell line, to allow optimization of the different sonicator parameters, including the power setting and the number of cycles. When the Chromatrap® technique is applied, effective shearing of primary cells and cell lines has been achieved using a water bath sonicator.
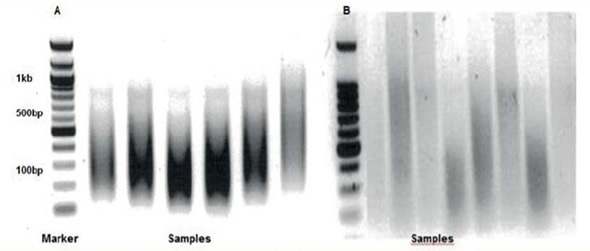
Figure 1. Optimal sonication and chromatin fragment length.
After optimal sonication conditions (Figure 1), uniform chromatin fragment lengths of between 100 and 500 bps should be viewed using agarose gel electrophoresis (A). Incorrect sonication will lead to variability in fragment length and diffuse smears. The two most important factors in enzymatic shearing are concentration of enzyme and lysis of cell membranes. To obtain optimal fragment lengths of 100 and 500 bp, the chromatin ratio must be optimized.
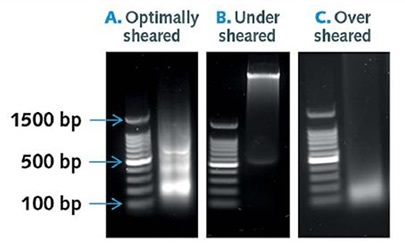
Figure 2. Optimally sheared, under sheared and over sheared samples.
To determine the optimum ratio of the sample, either a dilution series of enzyme can be performed or a concentration of enzyme can be selected and the digestion times adjusted. Using Chromatrap®, it only takes five minutes for 1U/5μg chromatin to provide fragments of optimal length.
After optimal sonication conditions, uniform chromatin fragments of 100 to 500 bps in length should be visualized using agarose gel electrophoresis (A). Poor sonication will give rise to differing fragment lengths and diffuse smears, with samples displaying fragment sizes between 100 and 1000 bp (B). Under digestion will result in fragment lengths above 500bps in size (B), while over digestion will lead to complete fragmentation to 200 bp (C), as shown in Figure 2.
In cases where cell membranes are not properly lysed, the enzyme will have restricted access to the chromatin. This can be monitored using a phase contrast microscope to make sure that all the nuclei are released before moving to enzymatic digestion. The samples can be incubated in the lysis buffer for a longer period of time. The sonication method can then be used if cells are still resistant to lysis even after longer incubation.
7. Use a ChIP Validated Antibody
For successful ChIP assay, high quality and specific ChIP validated antibodies are required. In order to ensure that ChIP methodology and chromatin preparation are appropriate, ChIP validated positive and negative antibody controls must be included.
8. Run Positive and Negative Antibody Control Always
Negative and positive antibody controls should be run alongside the test antibodies. This would provide an indication of how effective the immunoprecipitation step is, as well as ensuring that the chromatin preparation is adequate. The Chromatrap® premium chip kit provides a polyclonal antibody for the histone mark H3 as a positive control and IgG as a negative control.
qPCR-optimized primers are also included. Alternatively, a ‘mock’ ChIP reaction with no primary antibody can be used as a control to measure background levels. Besides antibody controls, a negative and positive gene target act as good controls for ensuring that enrichment of the antibody is selective.
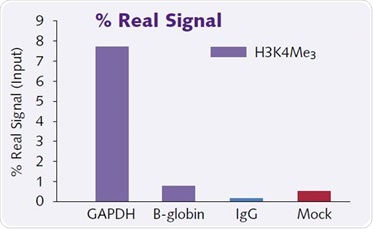
Figure 3. Graph shows high levels of enrichment (7.7% real signal) of H3k4Me3 onto a positive house-keeping gene GAPDH seen with Chromatrap®.
Figure 3 shows little to no enrichment for H3k4Me3 on to the negative target gene B-globin. Also shown are IgG and a no antibody (mock) control with 0.15 and 0.38% real signal. This demonstrates the sensitivity and specificity of the Chromatrap® ChIP assay.
9. Obtain Optimal Ratio of Antibody to Chromatin
When the antibody to chromatin ratio is incorrect, the signal-to-noise ratio may be compromised. An excess of antibody may saturate the ChIP assay and result in unspecific binding. An inadequate amount of antibody will mean insufficient chromatin binding occurs, therefore failing to provide an accurate representation of the antibody enrichment in the sample. The unique solid phase platform from Chromatrap® provides a greater surface area for antibody binding, which enables molecular mixing as well as reducing non-specific binding (Figure 4).
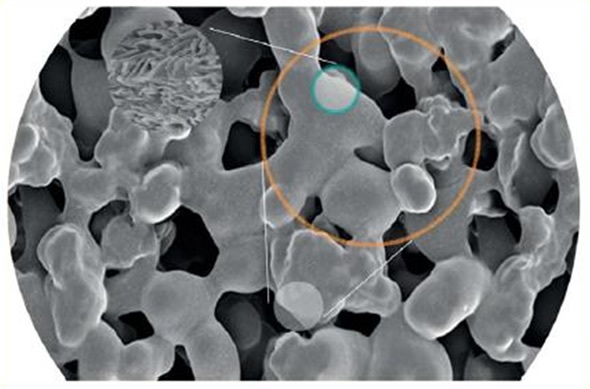
Figure 4. Chromatrap® offers an inert solid phase scaffold which increases the surface area for greater antibody binding, allowing for better immunoprecipitation and reducing non-specific binding.
Using Chromatrap® technology, small chromatin concentrations are used per IP (50ng-7μg). A 1:1 antibody:chromatin ratio is optimal at higher chromatin loadings (5μg and above), and a 2:1 ratio is optimal at lower chromatin loadings. These parameters allow the operator to save on antibody usage.
10. Optimise Lysis Buffer for Cells
A mild detergent is often found in lysis buffers that can precipitate out of solution if kept at cold temperatures. To ensure that buffer used to lyse cells is optimal, the solution should always be pre-warmed to 40°C and occasionally mixed to remove any precipitates. Before performing the lysis step, the buffer should be returned to room temperature and all precipitates re-dissolved.
During chromatin preparation, the amount of lysis buffer used is an important factor. Excess lysis buffer will mean an excess of detergent, which may have an inhibitory effect on antibody binding and downstream analysis. This would also give rise to a less concentrated chromatin preparation. Chromatrap® kits include optimized amounts of lysis buffer that can be used based on starting cell number (Table 2).
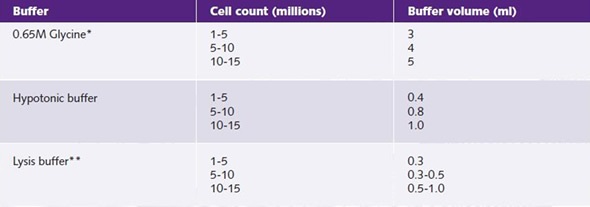
Table 2. Buffer, cell count, and buffer volume.
Conclusion
The three critical steps of chromatin preparation (fixation, lysis and shearing) should be fully optimized to ensure a successful ChIP assay. Using the aforementioned tips, positive ChIP results can be obtained quickly, easily and with efficient use of time and resources.
About Chromatrap®
 Chromatrap® is a product of Porvair Sciences, a wholly owned subsidiary of Porvair plc. We are one of the largest manufacturers of Ultra-Clean microplates, 96 well well filtration plates and Microplate handling equipment for life science and synthetic chemistry. With offices and Class VIII clean room manufacturing located in the UK, combined with a world-wide network of distributors and dedicated distribution hub in the USA, we pride ourselves on our continuous innovation, research and flexibility to meet customer demands. We offer OEM production and contract manufacturing through our North Wales facility.
Chromatrap® is a product of Porvair Sciences, a wholly owned subsidiary of Porvair plc. We are one of the largest manufacturers of Ultra-Clean microplates, 96 well well filtration plates and Microplate handling equipment for life science and synthetic chemistry. With offices and Class VIII clean room manufacturing located in the UK, combined with a world-wide network of distributors and dedicated distribution hub in the USA, we pride ourselves on our continuous innovation, research and flexibility to meet customer demands. We offer OEM production and contract manufacturing through our North Wales facility.
Our porous polymeric material, BioVyon™, whose chemical functionalisation can endow it with internal surface properties individually configured to capture and separate target species out of difficult mixtures, has opened up many possibilities in the field of BioSciences where molecules of interest such as DNA, RNA, proteins etc can be selectively pulled out of complex mixtures of biological origin. The materials have proven to be a remarkably good substrate for accepting novel chemistries such as the organically bound Protein A and Protein G in Chromatrap®.
Using our 25 years experience of microplate manufacturing, Porvair Sciences has now developed a high-throughput bead-free ChIP assay based on our filtration plates containing our Chromatrap chemistry. Chromatrap-96 enables large scale epigenetic screening to become a reality in many laboratories and eliminates many of the long and laborious steps previously undertaken in such work.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.