Introduction
Chromatin immunoprecipitation or ChIP is a useful method for studying interactions between DNA and proteins, and therefore improves understanding of gene regulation mechanisms.
During the ChIP process, it is important to prepare high-quality chromatin fragments ranging from 100 to 500 base pairs (bps) in length. These fragments are produced by restriction enzyme digestion or mechanical shearing.
The former process offers a cost-effective alternative to mechanical shearing because expensive equipment is not required. In certain situations, the shearing process is preferred over sonication.
Table 1 shows the advantages and disadvantages of the two different techniques used in chromatin preparation.
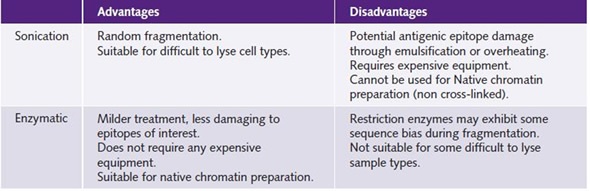
Table 1. Comparison of shearing methods for chromatin preparation.
Chromatrap® Enzymatic Shearing Kit for Preparation of ChIP-Grade Chromatin
The Chromatrap® Enzymatic Shearing Kit is useful for preparing high-quality chromatin for ChIP analysis. As evidence of this, the ChIP method was used to enrich high abundant Histone 3 (H3) signal at the GAPDH (Glyceraldehyde-3-phosphate) gene loci.
The Chromatrap® Spin Column Sonication Kit was used to prepare chromatin so that the quality of the chromatin could be compared between the different shearing techniques. The Chromatrap® Spin Column Sonication Kit and the Chromatrap® Enzymatic Shearing Kit were used to prepare high-quality chromatin that was sheared to optimal fragment sizes.
In both types of chromatin preparations, good target gene enrichment with a high signal-to-noise ratio was seen. The Chromatrap® Enzymatic Shearing Kit is a valuable tool for preparing high-quality chromatin quickly and without the need for high-priced sonication instruments.
Experimental Method
The human endometrial cell line Hec50 was used to prepare chromatin. According to the standard protocol, 1 million Hec50 cells were processed for chromatin isolation using the Chromatrap® Spin Column Sonication Kit and the Chromatrap® Enzymatic shearing Kit.
Cells were grown to approximately 80% confluency prior to cross-linking in 1% formaldehyde, quenching with glycine and subsequent collection in ice-cold PBS. Prior to re-suspension and lysis of cell membrane in Hypotonic Buffer, cells were spun down and the supernatant was removed. For Enzymatic shearing, the nuclei released were gathered by centrifugation and again suspended in digestion buffer to promote in-nucleus digestion of the chromatin.
To determine the approximate concentration, a 10µl aliquot of the nuclei suspension was first removed and lysed using a NanoDrop spectrophotometer. Depending on this concentration, a suitable amount of shearing cocktail was introduced into each nuclei suspension at a ratio of 1U/5µg chromatin and then incubated at 37°C temperature for a period of 5 minutes.
Using enzymatic stop solution, the reaction was stopped and suspensions were placed on ice. Nuclei were collected by centrifugation and lysed by adding Lysis Buffer. The suspensions were centrifuged to pellet cells and the supernatant comprising sheared chromatin stock was moved to a new microcentrifuge tube.
A 25µl aliquot of chromatin stock was taken, then reverse cross-linked and proteinase K digested to determine the concentration of stock and assess the quality of chromatin using agarose gel electrophoresis (Figure 1).
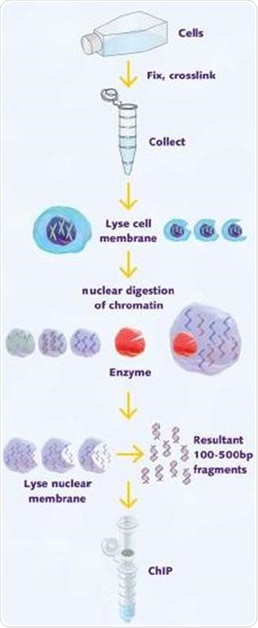
Figure 1. Schematic illustration of chromatin shearing by enzymatic digestion for use in ChIP.
In the case of chromatin prepared through sonication, subsequent to cell membrane lysis, a suitable amount of Lysis Buffer was used to release nuclei and the isolated nuclear fraction was separated using centrifugation. The chromatin was sheared to obtain suitable fragment lengths of 100 to 500bp (Figure 2).
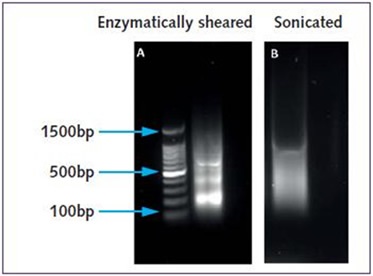
Figure 2. Agarose gel electrophoresis of Hec50 chromatin prepared using the Chromatrap® Enzymatic Shearing Kit and Chromatrap® Spin Column Sonication Kit. The typical ladder-like banding pattern observed after enzymatic digestion, demonstrate fragments of 200bp, 400bp and 600bp – ideal for ChIP using Chromatrap® ChIP columns or high throughput microplates. (1A). Uniform chromatin fragment lengths between 100 and 500bp visualized with sonicated chromatin (1B).
Immunoprecipitation Using Chromatrap® Spin Columns
For the analysis, core H3 was chosen as an antibody target. H3 is always present in chromosomes, therefore providing an abundant antibody target in the ChIP process. The GAPDH locus is expressed in all cell types and offers an abundant gene target.
Next, 50ng/µl working stocks in sterile distilled water were prepared to standardize chromatin stocks. Slurries were prepared using 1µg of chromatin and 2µg of relevant antibody. As per the standard procedure, the Chromatrap® Pro-A Spin Column Kit was used to perform the ChIP process.
Inputs were then prepared in parallel that contained 1µg of chromatin from each cell line. These samples were not subjected to ChIP enrichment, but were used for analyses. After elution, samples were then reverse cross-linked alongside the inputs, and proteinase K digested so as to release DNA for qPCR. Each combination of chromatin/antibody was performed three times to show the reproducibility of the enrichment using chromatin prepared with the kit.
qPCR was utilized to study the precipitation of GAPDH gene loci using antibody directed against the core H3. Precipitation of these loci utilizing non-specific IgG was also measured. The percentage of real signal was measured as a proportion of the chromatin and was normalized using the signal produced through non-specific binding of unspecific IgG.
Results and Discussion
Using Chromatrap® 96HT and Chromatrap® spin columns, sonicated chromatin obtained from primary cells and cancer cell lines was reproducibly enriched for low and high abundant targets. In order to show the utility of the Chromatrap® Enzymatic Shearing Kit for preparing high quality chromatin for ChIP analysis, chromatin was extracted from endometrial cell lines and then immunoprecipitated using Chromatrap® Pro-A spin columns.
When compared to the non-specific IgG, immunoprecipitation utilizing 1µg chromatin gave a 12-fold increase in specific H3 enrichment at the GAPDH promoter. This shows the high signal-to-noise ratio that results when chromatin is extracted using the Chromatrap® Enzymatic Shearing Kit.
For core H3, a high real signal was achieved, which was similar to that achieved when Hec50 chromatin was prepared using the sonication process (real signal of 4.56% of H3 at the GAPDH loci). Good reproducibility has been shown, demonstrating a low variability between samples, regardless of the starting cell number (Figure 3).
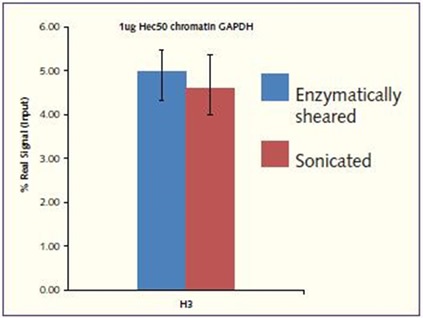
Figure 3. H3 signal enrichment at the GAPDH promoter. Using chromatin prepared using the Chromatrap® Enzymatic Shearing Kit and Chromatrap® Spin Column Sonication Kit, strong amplified signal was obtained at the GAPDH gene promoter following H3 IP. Excellent signal to noise ratio in both Hec50 chromatin preparations.
Conclusion
The Chromatrap® Enzymatic Shearing Kit is suitable for preparing high quality, ChIP-grade fragmented chromatin. The article has shown that excellent enrichment is possible, regardless of starting cell number, with similar GAPDH enrichment from immunoprecipitation of 1μg chromatin with H3 antibody for both enzymatically digested chromatin and sonicated chromatin. With a simple and rapid protocol, the Chromatrap® Enzymatic Shearing Kit offers a low-cost alternative to sonication.
About Chromatrap®
 Chromatrap® is a product of Porvair Sciences, a wholly owned subsidiary of Porvair plc. We are one of the largest manufacturers of Ultra-Clean microplates, 96 well well filtration plates and Microplate handling equipment for life science and synthetic chemistry. With offices and Class VIII clean room manufacturing located in the UK, combined with a world-wide network of distributors and dedicated distribution hub in the USA, we pride ourselves on our continuous innovation, research and flexibility to meet customer demands. We offer OEM production and contract manufacturing through our North Wales facility.
Chromatrap® is a product of Porvair Sciences, a wholly owned subsidiary of Porvair plc. We are one of the largest manufacturers of Ultra-Clean microplates, 96 well well filtration plates and Microplate handling equipment for life science and synthetic chemistry. With offices and Class VIII clean room manufacturing located in the UK, combined with a world-wide network of distributors and dedicated distribution hub in the USA, we pride ourselves on our continuous innovation, research and flexibility to meet customer demands. We offer OEM production and contract manufacturing through our North Wales facility.
Our porous polymeric material, BioVyon™, whose chemical functionalisation can endow it with internal surface properties individually configured to capture and separate target species out of difficult mixtures, has opened up many possibilities in the field of BioSciences where molecules of interest such as DNA, RNA, proteins etc can be selectively pulled out of complex mixtures of biological origin. The materials have proven to be a remarkably good substrate for accepting novel chemistries such as the organically bound Protein A and Protein G in Chromatrap®.
Using our 25 years experience of microplate manufacturing, Porvair Sciences has now developed a high-throughput bead-free ChIP assay based on our filtration plates containing our Chromatrap chemistry. Chromatrap-96 enables large scale epigenetic screening to become a reality in many laboratories and eliminates many of the long and laborious steps previously undertaken in such work.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.