Sponsored Content by PittconNov 11 2015
Alzheimer’s disease is a progressive neurodegenerative condition that causes dementia. In this disease, dementia symptoms such as memory loss and difficulties with language, problem-solving and reasoning gradually worsen until they eventually start to interfere with everyday life and basic tasks such as eating and drinking.
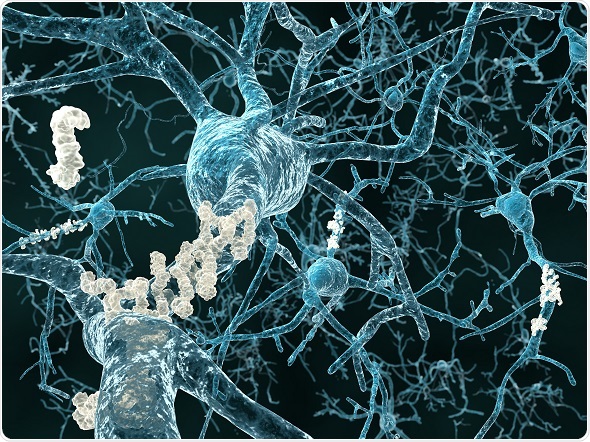
Alzheimers amyloid plaque - © Juan Gaertner / Shutterstock.com
Alzheimer’s affects more than 520,000 people in the UK and in the US, it will affect over 5 million people this year. The risk of developing the disease increases with age and it is most common among those aged 65 or older. Up to 5% of people with Alzheimer’s have an early-onset form of the condition that typically develops between the ages of 40 and 60. However, this is a rare condition caused by inherited mutations of single genes; mostly, people develop late-onset Alzheimer’s disease (LOAD), which has a much more complex pathology.
Understanding Alzheimer’s
Researchers have found that Alzheimer’s is partly caused by the build-up of abnormal structures in the brain called amyloid plaques. These are clumps of abnormally folded amyloid-beta protein (Aβ) that form on the outside of brain cells and disrupt cell-to-cell communication in the hippocampus. This region of the brain is involved with memory and spatial navigation.
Scientists are currently exploring the biological processes involved in Alzheimer’s and trying to translate their findings into diagnostic or therapeutic tools to help people with the condition. The fields of genomics and proteomics play an important role in answering biological questions about the disease and guiding research, diagnostic and therapeutic approaches.
Genomics
Advances in genomics have led to a revolution in research-based discoveries that have helped elucidate the complex pathology of Alzheimer’s. For example, the most influential genetic factor so far identified in studies of LOAD is an allele of the apolipoprotein E (APOE) gene called APOE4. In 2013, a study published in Nature combined several genomic techniques to identify key processes that explain the link between APOE4 and the development of LOAD.
Asa Abeliovich and colleagues examined whole-transcriptome data on gene expression levels in healthy APOE4 carriers and patients with LOAD. The researchers found that positive APOE4 status was associated with gene expression changes that resembled the LOAD profile, suggesting that APOE4 carriers have a certain set of gene expression changes in their brain that promotes LOAD. Further analysis revealed a set of potential key regulatory mediators including RNF219 and SV2A, which are known to play a role in the processing and transport of LOAD associated amyloid beta A4 precursor protein (APP).
A 2015 study by Hongtu Zhu and team examined whether whole genome SNP data combined with high dimensional whole brain imaging data could offer predictive values that could help identify people who are at risk of developing Alzheimer’s. Along with neurocognitive and clinical data, they assessed whole genome data and performed high dimensional MR imaging of 93 brain regions in 343 people with mild cognitive impairment and then followed the patients for 48 months. At the end of the follow-up period, 150 of the participants had developed Alzheimer’s disease. The researchers found that combining whole genome data with whole brain MR imaging data was significantly superior at predicting time to Alzheimer’s onset, compared with standard methods.

© Sergey Nivens / Shutterstock.com
Proteomics
The systematic study of the structure, function and interactions of proteins expressed in cells is often regarded as the next step following genomics. Investigating the protein spectrum of a cell and its biological function could potentially lead to the detection of diagnostic markers for Alzheimer’s and the development of therapeutics.
In 2012, AN Santos and colleagues described a flow cytometric approach for detecting and examining oligomers of Aβ in the cerebral spinal fluid (CSF) of 14 patients with Alzheimer’s and 12 healthy controls. The team found that CSF Aβ-oligomer levels were elevated in Alzheimer’s patients, compared with levels in the control group. Importantly, there was also a negative correlation between Aβ-oligomer levels and the Mini-Mental Status Exam score in patients with Alzheimer’s. The researchers therefore suggest that flow cytometry-based detection of Aβ-oligomers may be useful for assessing disease stage and the severity of dementia in Alzheimer’s.
Symposia at Pittcon 2016
This year’s Pittcon, the world’s largest annual conference and exposition for laboratory science, will take place between the 6th and 10th March 2016 in Atlanta, Georgia. The event’s impressive technical program will feature more than 2000 presentations. Among these, topics will include the use of ‘omics-based approaches as powerful investigative tools for exploring biological processes, including those involved in Alzheimer’s disease.
We will hear about the different levels at which Alzheimer’s can be understood, including at the genomic and proteomic level, and the advances that have led to higher throughput for studying complex systems and answering questions about the disease. The challenges involved in synthesizing information from these multiple levels will also be discussed, along with examples of approaches to understanding the different biological aspects of Alzheimer’s disease.
Among the 683 organizations exhibiting at this year’s Pittcon is Beckman Coulter Life Sciences, a leading and world-class company dedicated to advancing science through new discoveries and scientific breakthroughs. Offering a host of sophisticated products used in both academic and commercial laboratories, Beckman Coulter empower discoveries in biology-based research and development, including in the fields of genomics and proteomics. PolyLC will also be exhibiting, a leading provider of column-based solutions for the separation, isolation and analysis of proteins and peptides.
References
- Shatz C, et al. Human LilrB2 Is a β-Amyloid Receptor and Its Murine Homolog PirB Regulates Synaptic Plasticity in an Alzheimer’s Model. Science 2013;341:1399–1404
- Gotz J, et al. Alzheimer's disease models and functional genomics—How many needles are there in the haystack? Front. Physio. 3:320. doi: 10.3389/fphys.2012.00320
- Abeliovich A, et al. Integrative genomics identifies APOE ε4 effectors in Alzheimer's disease. Nature 2013;500:45–50
- Zhu H, et al. Predicting Alzheimer's Disease Using Combined Imaging-Whole Genome SNP Data. Journal of Alzheimer’s Disease 2015;46:695–702
- Simic G, et al. Update on the core and developing cerebrospinal fluid biomarkers for Alzheimer disease. Croatian Medical Journal 2014;55:347–65
- Santos AN, et al. Amyloid-β oligomers in cerebrospinal fluid are associated with cognitive decline in patients with Alzheimer's disease. Journal of Alzheimer’s disease 2012;29:171–6
About Pittcon
 Pittcon® is a registered trademark of The Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy, a Pennsylvania non-profit organization. Co-sponsored by the Spectroscopy Society of Pittsburgh and the Society for Analytical Chemists of Pittsburgh, Pittcon is the premier annual conference and exposition on laboratory science.
Pittcon® is a registered trademark of The Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy, a Pennsylvania non-profit organization. Co-sponsored by the Spectroscopy Society of Pittsburgh and the Society for Analytical Chemists of Pittsburgh, Pittcon is the premier annual conference and exposition on laboratory science.
Proceeds from Pittcon fund science education and outreach at all levels, kindergarten through adult. Pittcon donates more than a million dollars a year to provide financial and administrative support for various science outreach activities including science equipment grants, research grants, scholarships and internships for students, awards to teachers and professors, and grants to public science centers, libraries and museums.
Visit pittcon.org for more information.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.