In the field of biomedical research, accurate counting of cells is extremely important for ensuring both inter- and intra-lab reproducibility. Inaccurate cell counts have a negative impact not only on the reproducibility, but also on the potency and/or efficacy of cell therapy, disease diagnosis, the growth rate of regenerated tissues and various bioassays.
Traditionally, the gold standard for acquiring viable cell counts, has been to stain cells with a dye such as trypan blue, place them in a hemocytometer and count them manually under a microscope. Despite being a versatile and low-cost procedure, manual cell counting is a slow and labor intensive process. In addition, there is a risk of cross-contamination with the reusable hemocytometer, and different hemocytometer volumes, pipetting inconsistencies, and inter-use variation may lead to significant variations in results.
In contrast, the LUNA-II™ Automated Cell Counter from Logos Biosystems provides accurate, consistent and highly reproducible results at a reasonable cost. With this automated cell counter, the above-mentioned downsides of manual counting are eliminated.
The First Automated Cell Counter Fitted with a Liquid Lens – the LUNA-II™
The state-of-the art liquid lens in the LUNA-II™ Automated Cell Counter enables auto-focusing. This counter performs high-speed and accurate cell counts by using statistical observations of intensity characteristics in both live and dead cells. Compared with traditional optical lenses, the liquid lenses offer a number of advantages; they are long-lasting, compact, shock resistant, high-speed, offer a wide range of optical variation and consume less power.
The liquid lens and advanced counting algorithm also mean less variability in cell concentration and viability determinations. The technical variability in cell counting may arise when repeated measurements of the same single sample are made using the same device (intra) or made using different devices (inter). With the LUNA-II™, the advanced engineering and high quality control mean device-to-device variability is low and that intra- and inter-lab consistency and reproducibility are high.
Materials and Methods
Intra-deviation was tested after standard quality control procedures, using three LUNA-II™ devices. Inter-deviation was tested using seven of the devices. For all counts, the DEFAULT protocol of the LUNA-II™ was used.
Cell Lines and Reagents
A human cervical adenocarcinoma (HeLa) cell line was maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 units/ml penicillin/streptomycin. A human promyelocytic leukemia (HL-60) cell line was maintained in RPMI-1640 medium containing 10% FBS and 100 units/ml penicillin/streptomycin. LUNA™ Standard Beads and 0.4% trypan blue stain were used.
A series of cell suspensions with different viabilities was prepared by mixing live cells, cultured from exponentially growing cells, and dead cells, prepared through incubation of an appropriate number of cells at 70°C for 30 minutes.
Results
Linearity of Concentration Analysis of the LUNA-II™
The linearity of the LUNA-II™ was determined through measurement of the concentrations of serially diluted LUNA™ Standard Bead solutions. Figure 1 illustrates the high degree linearity exhibited by the LUNA-II™, as shown by R2 values of ³ 0.9999, irrespective of the level of experience. These results indicate the high accuracy of the LUNA-II™ in measuring particle concentration.
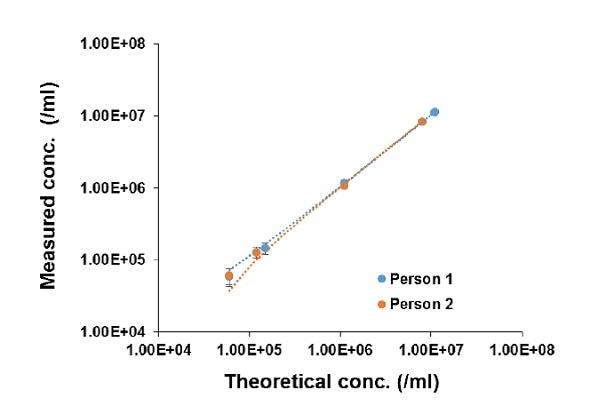
Figure 1. Concentration of Standard Beads determined using LUNA-II™ Data
The cell counting linearity of the LUNA-II™ was also determined using live cultured cell lines of HL-60 (suspension) and HeLa (adherent) cells. Before counting was performed, exponentially growing cells were serially diluted and then mixed with an equal volume of 0.4% trypan blue. Regardless of the cell type used, the LUNA-II™ again exhibited high linearity (R2 > 0.997) (Figure 2).
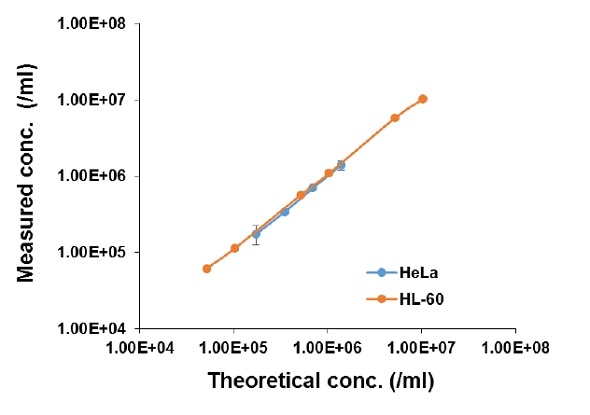
Figure 2. Determining cell concentration using the LUNA-II™
Linearity of Cell Viability of the LUNA-II™
The cell viability linearity measured using the LUNA-II™ was also determined using HL-60 and HeLa cells. As per the “Materials and Methods” described here, a range of cell viability solutions were prepared and the viabilities measured with the LUNA-II™. As shown in Figure 3, the viabilities closely matched the theoretical values, with R2 > 0.997.
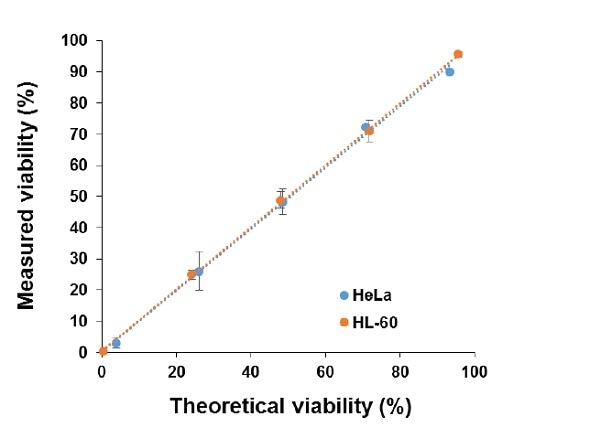
Figure 3. Cell viabilities determined using the LUNA-II™
Intra-and Inter-Device Deviation of the LUNA-II™
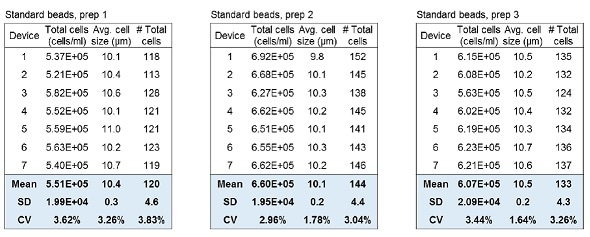
Reproducibility of cell counting is vital for subsequent applications of cultured cells. Here, two tests were performed to show the reproducible and consistent results that can be obtained with the LUNA-II™. The intra-device deviation was tested by loading LUNA™ Standard Beads on a LUNA™ Cell Counting Slide and counting them seven times for each device with three different devices. The inter-device deviation was tested by using seven different LUNA-II™ devices to count the beads.
The results in Table 1 indicate that repeated counting demonstrated similar results for total concentration, average cell size and the total number of cells. Table 1 also shows that the second and third devices exhibited great correlation. The slight differences seen with the first device were mainly caused by Brownian movement of the beads and/or evaporation.
Table 1. Intra-device deviation of the LUNA-II™
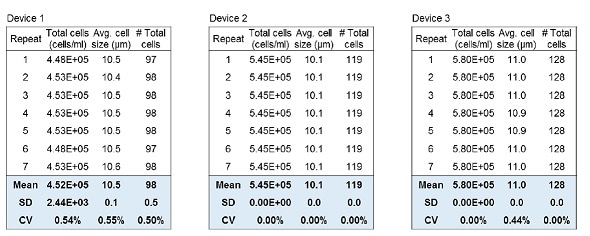
Table 2 shows the measurement of intra-deviation across seven LUNA-II™ devices. The coefficient of variance (CV) values of less than 5% for all the measurements indicates that the inter-device variability of the LUNA-II™ is significantly low.
Table 2. Inter-device deviation of the LUNA-II™
Conclusion
The main advantages of the LUNA-II™ Automated Cell Counter are as follows:
- It enables extremely accurate determination of cell concentration and cell viability.
- It ensures a high degree of linearity in cell concentration and cell viability in a wide working range.
- It provides a high degree of reproducibility and low technical variability.
References
- Sarkar 5, ISCT Webinar: Measurement assurance for cell enumeration. ISCT North American Legal and Regulatory Affairs (LRA) Committee. 2015. https://isct-beacon.app.box.com/isctwebinar29042015. Accessed 9 June 2015.
- Johnston G. Automated handled instrument improves counting precision across multiple cell lines. BioTechniques. 2010;48:325-7.
- Cadena-Herrera D, Lala JEE-D, Ramirez-Ibanez ND, Lopez-Morales CA, Perez NO, Flores-Ortiz LF, Medina-Rivero E. Validation of three viable-cell counting methods: manual, semi-automated, and automated. Biotech Rep. 2015;7:9-16.
- Tholudur A, Giron L. Alam K, Thomas T, Garr E, Weatherly G, Kulowiec K, Quick M, Shepard S. Comparing automated and manual cell counts for cell culture applications. BioProcess Int. 2006:4:28-34.
- Strober W, Trypan blue exclusion test of cell viability. Curt Protoc Immunol. 1997;A3,B.1.-A3.B.2.
- Lin H-C, Chen M-S, Lin Y- H. A review of electrically tunable focusing liquid crystal lenses. Trans Electr Electron Mater, 2011;12:234-240.
- Berge B. Liquid lens technology: principle of electrowetting based lenses and application to imaging. Proceed IEEE Int Conf Micro Electro Mech Syst 2005;227-230.

About Logos Biosystems, Inc.
Dedicated to the development and commercialization of innovative technologies to support life science research community. Founded at year 2008, Logos Bio is developing a series of lab automation systems and imaging instruments. The primary commercial target of Logos Biosystems is cell biology and molecular biology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.