The X-CLARITY™ Tissue Clearing System is a cutting edge advancement in the field of biomedical research, transforming biological tissue into a state transparent to light. This system removes lipids electrophoretically from the tissue-hydrogel hybrids. Lipids are fat molecules that are the main component of light scattering in biological tissues. The transformed tissue is labeled with probes such as fluorescent-labeled antibodies. After labeling, the tissue is infused and mounted in a refractive index (RI) matching medium so as to improve the optical clarity during 3D imaging.
In this case, the X-CLARITY™ Mounting Solution recently developed by Logos Biosystems was used as the RI matching medium. This is an excellent optical clearing agent for providing a 3D imaging environment with fluorescence microscopy and for preserving fluorescence signals in the labeled tissues for long periods.
The X-CLARITY™ Tissue Clearing System has overcome the disadvantages of the “do-it-yourself” design of older techniques such as CLARITY. This system ensures fast and reproducible clearing results of various biological tissues by providing uniform electric current and efficient control of temperature.
To perform 3D imaging, labeled tissue is usually placed in a commercial RI matching medium such as the FocusClear. However, FocusClear is expensive and not widely available. Logos Biosystems has therefore developed the X-CLARITY™ Mounting Solution as an alternative. This article compares the X-CLARITY™ Mounting Solution and the FocusClear for image quality and preservation of fluorescence signals in mouse brain slices.
Materials and Methods
Mouse Brain Tissue Preparation
A 12-week-old female ICR mouse was anesthetized with Avertin (250 mg/kg; Sigma, St. Louis, MO). Transcardial perfusion was carried out using ~30ml of phosphate-buffered saline (PBS) and ~10ml of 4% paraformaldehyde (PFA). The brain was further incubated with 4% PFA for 24h at 4°C and then incubated with Hydrogel Solution for another 24 hours at 4°C. Using a prototype of the X-CLARITY Polymerization System (Logos Biosystems), hydrogel polymerization was carried out for 3 hours under vacuum at 37°C. Following polymerization of the imbedded hydrogel, the brain was rinsed with the Electrophoretic Tissue Clearing Solution and then cut into 2mm-thick slices using the Brain Matrix for mouse (Ted Pella, INC, Redding, CA). The slices were placed in a Mouse Brain Slice Holder and the X-CLARITY Tissue Clearing System was used to electrophoretically clear the tissue for 2 hours at 37°C, 1.5A, and 30rpm.
After Immunolabeling
To carry out immunolabeling, the cleared brain slices were washed with 1X PBS overnight to remove SDS. The slices were then incubated in a buffer comprising 1:300 a-smooth muscle actin (Abcam, Cambridge, UK) and 1:300 collagen IV (Abcam) in 1X PBS supplemented with 0.2% Triton X-100 and 6% BSA for one day at 37°C, with gentle shaking. They were then washed for one day at 37°C in PBST (1X PBS containing 0.2% Triton X-100). Further incubation of the brain slices was performed with the Alexa Fluor 488-conjugated secondary antibody (1:200; for a-smooth muscle actin) for one day at 37°C, followed by PBST washing for one day. The brain slides were further incubated with the Cy3-conjugated secondary antibody (1:200, Jackson ImmunoResearch Laboratories, West Grove, PA; for collagen IV) for one day at 37°C, followed by PBST washing for one day.
Mounting and Imaging
Once immunolabeling was complete, each brain slice was cut into two pieces along the center line to enable comparison of the two mounting media (Figure 1). The X-CLARITY™ Mounting Solution was used for incubation of one half of the brain slice and FocusClear was used for incubation of the other half, for one day at room temperature. The brain slices were positioned on an imaging plate with a bottom of 0.17-mm thick cover glass. A confocal microscope (LSM710, ZEISS, Oberkoche, Germany) fitted with a 10X Plan-Neofluar lens was then used to detect fluorescence signal.
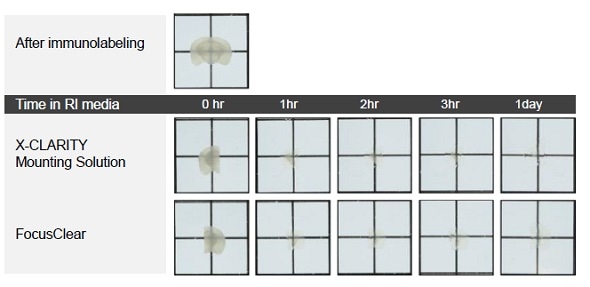
Figure 1. Comparison of cleared mouse brain slices incubated in the X-CLARITY™ Mounting Solution and the FocusClear.
Results
As shown in Figure 1, after immunolabeling, the brain slice became an opaque color due to lack of RI matching. After incubating the two halves of the brain slice for 1h in the X-CLARITY™ Mounting Solution and FocusClear, respectively, the optical clarity of both halves became evident (Figure 1). No significant difference was observed between the two RI matching media at this level.
After incubating both halves of the brain in the RI matching media for one day under identical instrumental settings for laser intensity power, gain, and pinhole size, confocal microscopy was performed to compare the fluorescence signals from each brain slice.
Figure 2A illustrates good detection of the red (collagen IV) and green (a-smooth muscle actin) fluorescence signals in the brain slice immersed in the X-CLARITY™ Mounting Solution. However, the fluorescence signals in the brain slice immersed in FocusClear were dim and difficult to read, as shown in Figure 2B. To obtain image quality similar to that obtained with the X-CLARITY™ Mounting Solution, the FocusClear required high levels of laser power and gain to compensate for low signals.
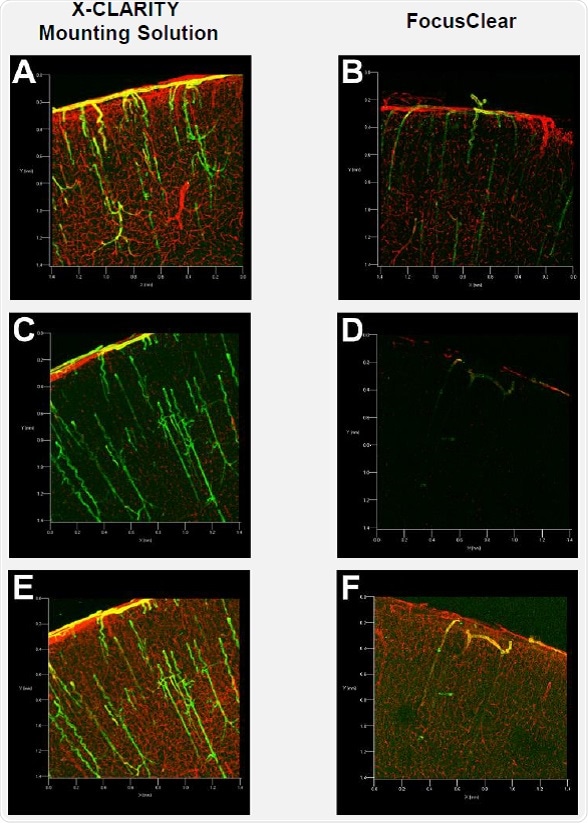
Figure 2. Confocal fluorescence microscopy of mouse brain slices
In order to determine whether the fluorescence signals are maintained in the tissues after extended incubation in the mounting media, the same brain slices were imaged after two weeks of incubation. Confocal fluorescence microscopy was carried out under the same instrumental settings used for imaging after the one-day incubation period. Surprisingly, both the red and green fluorescence signals were not detected in the brain slice immersed in FocusClear (Figure 2D). On the contrary, high levels of green and red fluorescence signals were detected in the brain slices incubated in the X-CLARITY™ Mounting Solution (Figure 2C).
The instrumental settings were changed for maximum detection of fluorescence signals in each brain slice. Comparable green and red fluorescence signals were detected in the brain slice incubated in the X-CLARITY™ Mounting Solution (Figure 2E), whereas the green fluorescence signals detected in the brain slice incubated in FocusClear were very limited (Figure 2F).
The resulting data suggest that although the two mounting media do not differ in terms of optical clearing (Figure 1), the detection and preservation of fluorescence signals differs significantly. Therefore, the X-CLARITY™ Mounting Solution is suitable for detecting fluorescence signals in immunolabeled, cleared tissues at a relatively low level of laser power. High-level laser power may expedite photobleaching of fluorescence molecules, particularly in deep tissue 3D imaging with confocal fluorescence microscopy. Therefore, efficient imaging with low-level laser power is valuable for performing 3D volume imaging with confocal fluorescence microscopy. Also, the X-CLARITY™ Mounting Solution allows parallel processing of multiple samples for later imaging because the fluorescence signals in immunolabeled tissues are preserved for long periods.
Conclusion
The main advantages of using the X-CLARITY™ Mounting Solution are described below:
- It enables efficient RI matching of cleared tissues following immunolabeling.
- It provides better conditions for 3D volume imaging of tissues with confocal fluorescence microscopy at a low level of laser power, thereby reducing photobleaching.
- It preserves comparable fluorescence signals for a minimum of two weeks.
Furthermore, the X-CLARITY™ Mounting Solution offers an exceptional alternative to the existing solutions because it is affordably priced, compared with the commercially available mounting medium FocusClear.
References
- Richardson, D. S., Lichtman, J. W. Clarifying tissue clearing. Cell 162, 246-257 (2015).
- Hama, H., et al. ScaleS: an optical clearing palette for biological imaging. Nat. Neurosci. 18, 1518-1529 (2015).
- Chung, K., et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332-337 (2013).
- Chung, K., Deisseroth, K. CLARITY for mapping the nervous systems. Nat. Methods 10, 508-513 (2013).
- Yang, B., et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 158, 945-958 (2014).
- http://forum.claritytechniques.org/.
- Marx, V. Microscopy: seeing through tissue. Nat. Methods 11, 1209-1214 (2014).

About Logos Biosystems, Inc.
Dedicated to the development and commercialization of innovative technologies to support life science research community. Founded at year 2008, Logos Bio is developing a series of lab automation systems and imaging instruments. The primary commercial target of Logos Biosystems is cell biology and molecular biology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.