Confocal microscopy, or more accurately confocal laser scanning microscopy (CLSM), is a derivation of optical microscopy that allows both the optical resolution and contrast of micrographic images to be increased.
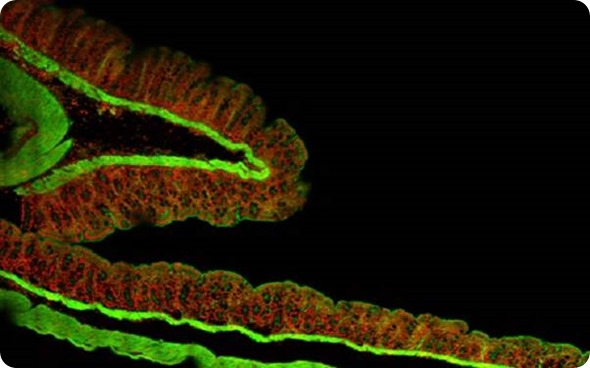
Figure 1. Montage of 2 mm x 1.5 mm area at 60x, pancreas tumor section.
This is accomplished largely by the use of a spatial pinhole at the confocal plane of the sample to block out-of-focus light rays, providing a very controlled depth of focus.
The microscope forms an image from any photons of light that originate from the plane of focus from the sample, which results in an improved signal-to-noise ratio compared to widefield imaging techniques [1].
The advantage of confocal microscopy to collect serial images at precise depths of the sample is known as optical sectioning. The power of confocal microscopy is enhanced largely if coupled with the ability to change the focal planes and optically section through a specimen.
Collecting at discrete focal planes provides a three-dimensional fluorescence profile allowing for improved spatial context for analyses. Combining the optical sectioning with newer high-speed confocal technology, this volumetric imaging can be performed quickly to capture fast biological dynamics.
This is particularly useful in cell biology for producing in vivo high-resolution images of labeled process components in living cells and also for examining processes occurring in and around cell membranes [3].
The following studies take advantage of a modern confocal technology known as “swept-field confocal” scanning. The swept field concept parallelizes the traditional single-point scanning confocal by scanning 32 points of light concurrently for fast, high-resolution scanning.
The researchers also utilized the slit (line) scan confocal modes of the swept-field instrument to achieve even higher speed imaging.
Endosome transport live images
Charcot-Marie-Tooth2b (CMT2b) is an axonal form of a human neurodegenerative disease that specifically distresses sensory neurons [4].
The disease is manifest in the Rab7 gene by five missense mutations. Rab7 is actually a small GTPase involved in endosome conversion and lysosome metabolism.
Transgenic zebrafish were produced with the required Rab7 mutations and by using confocal microscopy to examine Rab7 activity it was possible to produce high-speed, high-resolution imaging of endosome transport in vivo.
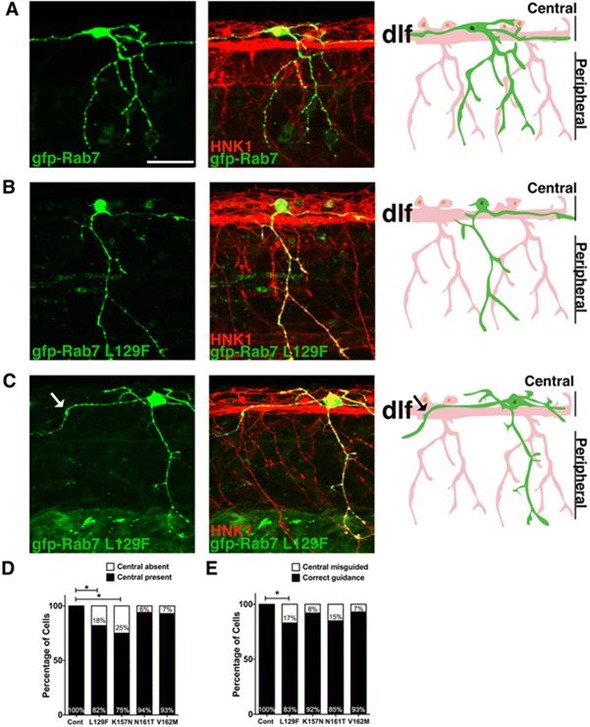
Figure 2. “Central axon guidance errors in sensory axons expressing CMT2b Rab7 mutants” from O.Y. Ponomareva, K.W. Eliceiri and M. C. Halloran, Charcot-Marie-Tooth 2b associated Rab7 mutations cause axon growth and guidance defects during vertebrate sensory neuron development, Neural Development (2016) 11:2, DOI 10.1186/s13064-016-0058-x. licensed under CC BY 4.0
The live high-speed (time lapse) imaging of endosomal trafficking was performed on a Bruker Opterra swept-field confocal microscope equipped with a Nikon CFI Plan Apo VC 60x oil immersion objective [4].
The live images were possible because of the Opterra’s Piezo Z-focus and they showed that CMT2b-associated Rab7 variants actually cause reduced vesicle speeds.
It was suggested from these observations that the altered transport of endosomes might well underlie axon development defects and provide the expression of this debilitating condition.
Confocal microscopy was central to the conclusions of this study as defects in axon development were a previously unrecognized component of CMT2b disease [4].
Biological membrane repair after damage
Cell damage elicits a repair response in the membrane, which quickly protects the integrity of the cell. The mechanism of this repair process has been the subject of much contention and in this study the process can be seen first-hand using images produced by confocal microscopy.
The mechanism of resealing the membrane has been thought to involve the formation of a patch from a rapid fusion of intracellular compartments at the wound site (patch hypothesis), however patching has never been directly observed [5].
In this study, the membrane dynamics of wounded Xenopus laevis (frog) oocytes at high spatiotemporal resolution were observed. The study presented certain problems that could only be addressed by using fluorescent visualization and confocal microscopy techniques [5]. These included:
- Patching occurs quickly, less than one second post damage
- Finding an accurate and controlled way to create a wound to the animal
- The wound is a moving target since actomyosin-powered contractions bring the edges of the wound together about 30-60s following the damage event
These challenges were overcome by using:
- High-speed laser scanning of the swept-field technology
- Simultaneous photoablation using the Opterra photoactivation/photoablation module while imaging
- Fast Z stacks using the piezo Z stage to collect volumetric information pre- and post-wounding
The study conducted using an Opterra multipoint confocal system shows the utility of confocal microscopy and how the cell membrane patching process could for the first time be observed in real-time.
The images showed how cell damage triggers a proliferating fusion of intracellular compartments, generating a barrier to limit the influx of extracellular dextrans. The patch formation then proceeds by compound exocytosis, local accumulation, aggregation of vesicles, and rupture of compartments facing the external environment.
The wound area also attracts a range of repair compounds such as annexin A1, dysferlin, diacylglycerol, active Rho, and active Cdc42, and there is an increase in the concentration of calcium in the area.
The confocal imaging process has shown how cell wounding is accompanied by a notable amount of spatial patterning and how important membrane dynamics are to retaining cell integrity.
Primary cilia as calcium ion responsive mechanosensors
It is generally assumed that primary cilia, the non-motile, hair-like protrusions on the surface of cells, are able to sense mechanical force courtesy of their calcium permeable ion channels.
This hypothesis has supported a range of biological responses, from control of left–right axis determination in development of embryos to the adult progression of polycystic kidney disease and also the progressive mechanism of some cancers [6].
The antenna-like structure of primary cilia has naturally pointed to the assumption that they sense the surrounding environment, by using their calcium-permeable ion channels.
This study has provided evidence for the complete lack of mechanically induced calcium increases in primary cilia and stereocilia in specific tissues, such as kidney epithelia and inner ear sensory epithelia [7].
The study used a Ca2+ imaging technique to examine the flow of calcium ions during mechanical stimulation. The tissues used were from a transgenic mouse whose tissues were modified to incorporate a calcium indicator (Arl13b–mCherry–GECO1.2).
The real-time responses were observed under an upright Nikon Ni-E microscope equipped with an Opterra swept-field confocal imaging system from Bruker and a Photometrics Evolve 128 EMCCD camera.
To capture the dynamics of the Ca2+, an incredibly high frame rate confocal system was required. The Opterra swept-field confocal enabled fast imaging of 500–1,000 frames per second in low-light conditions, allowing tissues to be illuminated in sequence by excitation laser-lines 488 nm (GECO1.2) and 561 nm (mCherry).
Swept-field confocal imaging was performed in slit mode (35 μm) to increase light delivery to the camera and avoid photobleaching. The imaging of calcium flows in cilia bearing epithelia concluded that mechanosensation, if it occurs in primary cilia, does not invoke calcium signaling.
Bruker Opterra
Confocal microscopy confers the ability to optically section a specimen and isolate light coming from a confined slice when forming an image. When coupled with advanced high-speed scanning technology and fast Z mechanics, it is an ideal tool to study real-time biological processes in multiple dimensions.
The Bruker Opterra confocal system offers these capabilities and is shown to be a flexible instrument, allowing a wide range of unique adjustments to balance settings for speed, resolution, and fluorescence intensity.
These studies demonstrate how this technology enabled cell biologists, with relative ease, to capture relevant biological events and formulate insightful conclusions.
References
- D. Semwogerere and E.R. Weeks, ‘Confocal Microscopy’, 2005, published in the Encyclopaedia of Biomaterials and Biomedical Engineering, Taylor & Francis.
- V. Prasad, D. Semwogerere, E.R. Weeks, ‘Confocal microscopy of colloids’, 2007, J. Phys.: Cond. Mat. 19, 113102.
- Handbook of biological confocal microscopy, edited by James B. Pawley, 2nd ed., Plenum Press, 1995.
- O.Y. Ponomareva, K.W. Eliceiri and M. C. Halloran, Charcot-Marie-Tooth 2b associated Rab7 mutations cause axon growth and guidance defects during vertebrate sensory neuron development, Neural Development (2016) 11:2, DOI 10.1186/s13064-016-0058-x.
- N. R. Davenport, K. J. Sonnemann, K.W. Eliceiri and W. M. Bement, Membrane Dynamics during Cellular Wound Repair, Mol Biol Cell. 2016 Jul 15;27(14):2272-85. doi: 10.1091/mbc.E16-04-0223. Epub 2016 May 25.
- M. Delling, A. A. Indzhykulian, X. Liu, Y. Li, t. Xie, D. P. Corey and D. E. Clapham, Primary cilia are not calcium-responsive mechanosensors, Nature 531, 656–660 (31 March 2016) doi:10.1038/nature17426.
- K. Zimmerman, and B. K. Yoder, Snapshot: sensing and signaling by cilia, Cell, 161, 692–2.e1 (2015).

About Bruker Nano Surfaces
Bruker manufactures world class atomic force microscopes and other nano technologies that incorporate the very latest advances in AFM techniques, including the revolutionary ScanAsyst™ AFM imaging mode and the PeakForce QNM® atomic force microscopy imaging mode to ideally suit a wide array of application areas, from biology to semiconductors, from data storage devices to polymers, and from integrated optics to measurement of forces between particles and surfaces.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.