Table of Contents
Introduction
Assessment of MI Levels
Impurities That Are Added
Impurities That May Have Formed
Detection Techniques
Summary
Presently ICH M7 and other guidelines give an overview of evaluation and assessment of limits of pharmaceutical impurities categorized as mutagenic. These could be degradation products of pharmaceutical processes, known excipients, or other environmental factors. As the levels of genotoxic impurities remain well below the limits described for common impurities in ICH Q3A guidelines, they need techniques capable of analyzing and measuring quantities in ppm or ppb. In this write-up, various techniques for detecting mutagenic impurities are described.
Introduction
Investigational new drugs need to be safe and effective. Safety requirements for CMC have been refined a lot in last few years. Specifically, assessment of drug product and active ingredient impurities in relation to manufacturing, and container closures are explained in USP, ICH guidelines, and other guidance by various regulatory agencies. The guidelines for trace metals and mutagenic impurities advise strict check on such impurities. Assessment of possible mutagenic impurities in drug products and new drug substances in order to minimize carcinogenic risk is outlined in ICH M7 guidance. A major challenge while measuring mutagenic impurities (MI) is the need for very low levels of detection.
The terms “carcinogenic” and “genotoxic” in the early industry articles and guidances were replaced with the term “mutagen” in the M7 guidance issued in 2015. The noteworthy difference is that a genotoxin may not be a mutagen. A mutagen is defined as below:
Anything that causes a mutation (a change in the DNA of a cell). DNA changes caused by mutagens may harm cells and cause certain diseases, such as cancer. Examples of mutagens include x-rays, certain chemicals, ultraviolet radiation, and radioactive substances.
Assessment of MI Levels
Levels of non-mutagenic impurities in drug substances are typically evaluated at 0.05% weight/weight levels using relative peak area or other standard detection techniques as per ICH Q3A guidelines. Recommended thresholds are evaluated by dose duration and daily intake. For a concentration below 10 ppm, these limit MIs to below 1.5 µg per day. Thus, a technique with 70-fold lower limit of detection may be needed, as described in Table 1. Introduction of MIs can be viewed, in a way by categorizing three primary sources with differing detection complexities according to the source of the MI.
Table 1. Comparing Q3A and M7 levels.
Therefore, it is evident from Table 1 that the MIs to be quantitated may need techniques with much higher sensitivity compared to standard Q3A impurities at 30% TTC (Threshold of Toxicological Concern) and at 0.05% level..
Two sources of mutagenic impurities are discussed here: impurities that are added and impurities which may have formed in the matrix. The third source, environmental MIs, also referred to as leachables, are not covered here, as they are typically evaluated using independent programs.
Impurities That Are Added
Discovering “things that are added” is relatively easier than “things that may have formed.” Both need an initial evaluation. For example, knowledge of addition of an acid chloride at step 3 of the synthesis, can suggest a straightforward approach for analysis of sample, and extrapolation of the detection characteristics. The available toxicological data may also simplify the analysis.
A detection technique and a separation technique are needed for assessment of intermediate or the final drug substance for presence of the added MI. This raises some important questions:
- Is the compound volatile?
- What is the required level of detection according to TTC?
- Does the current technique detect the MI, and if so, what is the level of detection?
- What is the anticipated ionization pattern of the MI? Is it relevant for MS?
- Can derivatization be considered based on the reactivity of the MI?
In general, impurities that are added are precisely of higher chemical reactivity and this should be leveraged during method development when accuracy is evaluated by spiking API into samples. For example, impurities of alkyl halides are reactive with amines and have been noted in GC headspace analysis to influence accuracy of recovery studies.
Impurities That May Have Formed
Evaluation of degradation products that alert for mutagenicity is more complicated than the added MIs. Additional efforts may be needed in case the Q3A(R2) or other process finds a degradation product with toxicological potential in the sample, such as described in Figure 1. There is a subtle difference between the Q3A(R2) decision tree and the note in the diagram mentioning, “Lower thresholds can be appropriate if the degradation product is unusually toxic.” This addresses degradation products but, does not indicate the requirement for identification. The decision tree indicates an alternative to reduce degradation product below the level of detection, therefore no further action is warranted. However, the mention of unusual toxicity of degradation product in the note suggests prior identification. That is, how can one evaluate toxicity of an unidentified degradation product? For assessment of mutagenic impurities, M7 is a better guidance compared to Q3A R2.
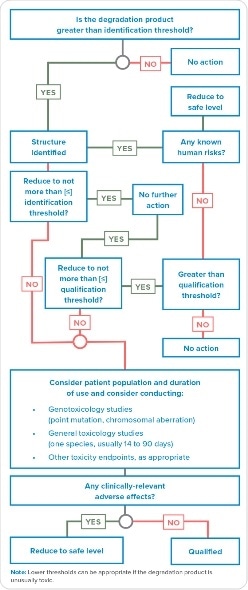
Figure 1. A Q3B(R2) decision tree for the identification and qualification of a degradation product
Following is an example of a worst-case situation:
- A possible degradation product of concern is identified by M7-like assessment in API sample, or there are two active ingredients and many excipients in the corresponding drug product.
Further evaluations such as purposeful stressing of drug substance to analyze the presence of an alerting structure in silico analysis, and a bacterial assay may be considered. Following additional questions need to be asked in addition to those for the added MIs:
- Is synthesis of degradation product and/or isolation needed to validate the absolute structure, provide material for in vivo studies and the analytical reference material?
- Should this product be assessed in complex drug products during long term stability studies?
One scenario that may occur is the presence of in silico functional group in the primary structure such as a substituted aniline that may indicate MI. Any known degradation product with aniline substructure can clearly give in silico alert. It is generally accepted that if a parent molecule is shown to be non-mutagenic, then the degradation products would also follow the pattern. However, it is advisable to carry out a risk assessment. ICH mentions that M7 is not relevant for advanced cancer drugs.
Detection Techniques
Some options for detection are more promising than others when there is a need to measure low levels of impurities. Table 2 indicates the general sensitivity of various detectors where the value of UV detector is assumed as 1, and the relative values of other detectors are mentioned. Thus, an electrochemical detector is generally 10 times more sensitive than UV detector. Note that these sensitivities are also compound dependent.
Table 2. General Sensitivity Overview-HPLC Detectors
As shown in Table 3, mass spectrometry detection has relatively superior sensitivity and also the identification potential. For example, single ion monitoring trap MS with instruments such as a Q Executive® Orbitrap has low quantitation limit in a complex matrix and is very helpful in screening and monitoring MIs.
Table 3. GC Detector Sensitivity4
While evaluating and quantitating MIs, it is essential to take inputs from toxicology, analytical, synthesis, and manufacturing experts to apply a strategy that is compound specific with constant evaluation during drug development.
Summary
- MIs may arise from three sources:
- Impurities that are added (including in-process impurities)
- Environmental impurities
- Degradation products
- Various detectors and techniques are available
- Low level detection capabilities are needed for profiling mutagenic impurities
- Newer MS technique is a useful tool for identification and quantitation
- The difficulty in identifying or quantitating MIs is proportional to whether the MI is a known entity or not, its compound properties, and the level of detection needed.
References:
- M7(R1) Addendum to ICH M7: Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. Retrieved from https://www.ema.europa.eu/en/ich-m7-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit-potential-carcinogenic-risk-scientific-guideline Retrieved from, https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=601170
- ICH Q3A R2, Guidance Impurities in New Drug Substances
- NCI Dictionary of Terms, Mutagen. Retrieved from https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=601170
- Hutchinson J. et al., (2011). Investigating universal detection systems for the analysis of pharmaceutical preparation. Retrieved from http://www.cosmoscience.org/

This information has been sourced, reviewed and adapted from materials provided by EAG Laboratories.
For more information on this source, please visit EAG Laboratories.