Proper quality measures are vital in all areas of clinical laboratory testing to prevent or avoid carryover between samples, as well as to minimize the risk of false-positive results. The sample preparation stage is one area that is often overlooked.
The shift from column processing to 96-well-based, high-throughput sample preparation is a growing trend in clinical laboratory testing. Samples can be processed more quickly, and various automated systems have been designed for processing 96-well plates.
When working with highly volatile compounds in samples that vary widely in concentration, great care must be taken to prevent false-positive outcomes for wells adjacent to one another.
The aim of this article is to explain easy practical approaches, hints, as well as tips to reduce or prevent cross-well contamination or “cross-talk” in high-throughput 96-well-based sample preparation methods.
While making attempts to prevent or remove the chances of cross-talk, it is essential to consider all aspects of the sample preparation procedure. Sample preparation hardware (for example, extraction and collection plates), processing equipment and conditions (such as evaporation), and method parameters (for example, solvents and volumes) are crucial aspects to improve.
Extraction and collection plate design
Correct penetration of Luer tips (plate outlet nozzles) into collection plate wells
Several styles of 96-well extraction plates (including SLE, SPE, protein precipitation, as well as other sample preparation techniques) are available in the market, and differences in their design could have a vital role to play in the degree of potential cross-well contamination.1
Extraction plates could vary in both the length of the Luer tip and the distance between the plate sealing edge (where the plate rests at the time of processing; refer to Figure 1) and the tip of the Luer. This influences the outlet nozzle’s penetration into the collection plate at the time of processing.
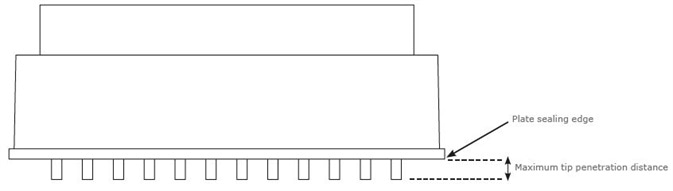
Figure 1. Profile of a 96-well extraction plate. Image Credit: Biotage
When sample preparation procedures are carried out, the extraction solvent flows via the wells (under gravity, vacuum, or positive pressure) and goes out through the Luer. Under specific conditions, a tiny burst of fine droplets released as an aerosol can develop as the well empties (sputtering).
This risk tends to grow as solvents with lower surface tension (or more volatile solvents) are used, with a further increase in risk when a vacuum is used instead of positive pressure for processing the plate.
Irrespective of the processing equipment (manual or automated, positive pressure, or vacuum-based) used, it is crucial to guarantee that there is enough penetration of the Luer tips into the wells of the collection plate.
The Biotage series of 96-well extraction plates (such as the base plates that can hold up to 96 separate tabless 1 mL columns), processing equipment (Biotage® Extrahera™ SLE and SPE Automation System, Biotage® Pressure-96 Positive Pressure Manifold, and Biotage® VacMaster™-96 Vacuum Manifold), and collection plates function collectively to guarantee the proper fit and easy sample processing (Refer to the Sample Processing section).
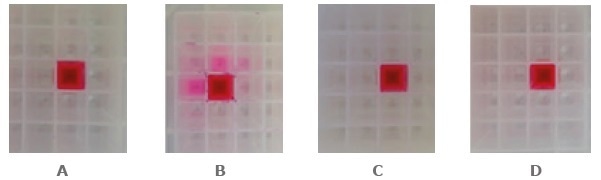
Figure 2. Impact of optimized Luer tip penetration on adjacent well contamination when processing SPE plates under different conditions. (A) Vacuum (VacMaster™-96) with an optimized spacer. (B) Vacuum (VacMaster™-96) with a non-optimized spacer. (C) Positive pressure (Biotage® Extrahera™). (D) Positive pressure (Biotage® Pressure + 96). Solvent: MeOH containing dye as a marker. Image Credit: Biotage
Length of the Luer tip and depth of penetration into the collection plate is the most crucial parameters for contamination reduction. However, other parameters like the diameter of the Luer opening might also influence the performance under certain conditions.
Other aspects of collection plate design to consider
- Users should always use a collection plate that has enough volume to contain the needed volume of elution solvent so that wells are not over-filled
- While mixing the sample in a 96-well plate, users must ensure that enough headspace is available to enable liquid displacement
Sample processing
Positive pressure versus vacuum processing
The use of positive pressure to process 96-well plates instead of vacuum can help minimize the risk of cross-talk because of the reduced risk of aerosol formation (sputtering) at the time of solvent elution. Biotage provides a series of both manual and automated processing systems, which have been upgraded for 96-well processing.
Automated sample processing: Biotage® Extrahera™ automation system
The Extrahera™ instrument can be employed for processing 48- and 96-well extraction plates, together with 96 separate 1 mL tabless columns.

Figure 3. Image Credit: Biotage
Moreover, the system can process 24 x 1, 3, or 6 mL columns that are held in a regular microtitre plate footprint. The instrument includes several features developed to avoid sample cross-contamination through the entire sample preparation technique.

Figure 4. Image Credit: Biotage
- The positive pressure system reduces sputtering (or aerosol formation) by regulating processing flow to prevent a considerable increase in solvent flow rate as the well empties. The highest flow restriction is in the positive pressure processing head, which helps ensure constant flow rates between wells or columns at the time of processing. Besides, integrated high capacity volumes can be allocated to each consumable, thereby reducing the probability of contaminating the sealing mat on the positive pressure head by over-filling while loading the sample.
- Before every operation in the technique, the carousel moves the flow via the plate (for directing waste) and the collection plate (or tube rack if processing columns) into the exact position. Upon being located, the plate lifter lifts the collection plate or rack to a place directly below the extraction column or plate and guarantees correct Luer or well penetration.
- Waste is directed to an external collection reservoir via the flow-through plate. The flow-through plate avoids contamination of adjacent Luers by droplets. The flow-through has been designed such that when it is used together with the lifter, it prevents Luer-to-Luer contamination on columns or well plates. At the time of elution, contamination of adjacent collection wells or tubes due to spluttering or splashing is also prevented with the help of flow-through plate.
- Aspirate and dispense heights of the pipette tip can be tuned for each consumable on the system, and therefore, safe reuse of the reagent pipette tip is possible without any risk of contamination.
- The system does not transfer from the sample plate or tube to the incorrect well or column while loading consumables, which guarantees sample traceability. Moreover, a pipette tip can never be reused after sample dispensation on a subsequent different sample.

Figure 5. Image Credit: Biotage
Manual positive pressure processing: Biotage® PRESSURE+ 96
Biotage® Pressure+ 96 manifolds offer constant positive pressure and parallel processing for 96-well plates. The systems employ an even positive pressure flow to move both high- and low-viscosity liquids via 96-well plates and up to 96 tablets 1 mL columns held in a uniquely designed base plate.
96-well plates
While processing with the Pressure+ 96, the extraction plate is placed directly on top of the collection plate, which guarantees optimum Luer tip penetration.
Tabless 1 mL columns
The base plate retains the columns safely on top of the collection plate and prevents any lateral movement. It has been developed to enable the most optimum Luer penetration into the collection plate.
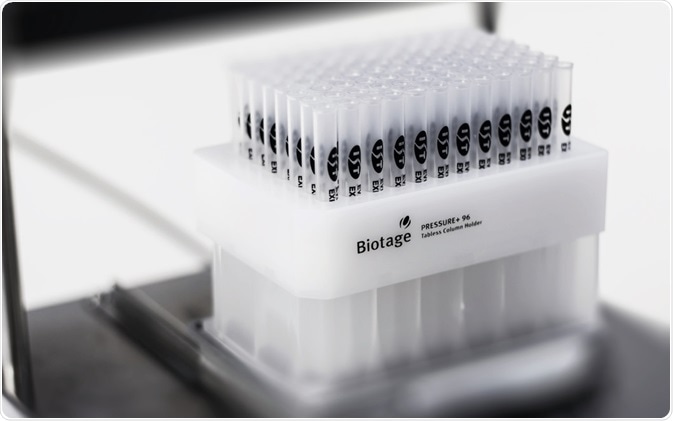
Figure 6. 96 x 1 mL tabless columns on Biotage® Pressure+ 96. Image Credit: Biotage
Manual vacuum processing: Biotage® VacMaster™-96
The VacMaster-96 manifold has been engineered to enable proper penetration of the extraction plate Luer tips into collection plates at the time of processing. Spacers are employed to accommodate extraction and collection plates of several designs. These ensure accurate positioning of the Luer tips while preventing any vacuum loss.
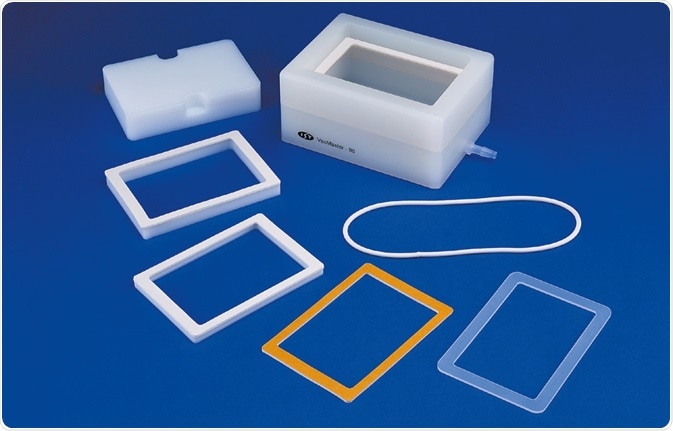
Figure 7. Biotage® VacMaster™-96 spacer options for extraction and collection plates to allow Luer tip position optimization. Image Credit: Biotage
Collection plate compatibility
The height of collection plates from different suppliers can vary. The Biotage® VacMaster™-96 features spacer inserts to enable different designs to be used without compromising the penetration of Luers.
Tabless column base plate
The tabless column base plate from Biotage (that can hold up to 96 tabless 1 mL columns, where unused positions are sealed with sealing tape) has been engineered to ensure sufficient Luer penetration even without using spacers for use on the VacMaster-96.

Figure 8. Image Credit: Biotage
Evaporation
In general, sample extracts should be evaporated after commonly used sample preparation methods, for instance, to decrease the volume or increase concentration, or to exchange a solvent with another one that is more compatible with the next analytical method.
Extracts are often filled in a 96-well collection plate at the time of evaporation and processed under a regulated flow of nitrogen or air. Several approaches can be used to prevent or remove cross talk related to evaporation.
Biotage® ACT plate adapter
“Hotspot” carryover is a phenomenon noticed in assays that involve forced evaporation with some equipment available in the market. In the evaporation stage, a proportional amount of analytes that exist in an extracted sample can contaminate adjacent wells by being carried with the volatilized solvent (see Figure 9).
If the analyte concentration in the “hotspot” well is significantly higher compared to that in the surrounding wells, there are higher chances of a clinically significant change in quantitation of the samples in the surrounding wells and thus possibly detrimental clinical implications.
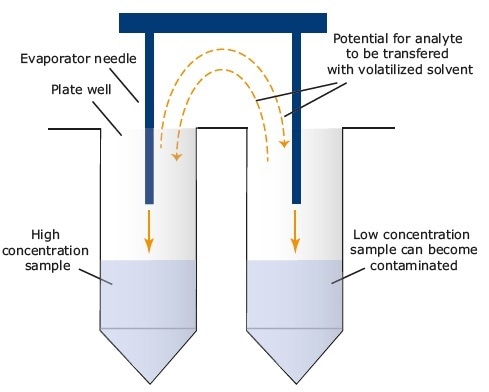
Figure 9. The mechanism for “hot spot” cross-talk during evaporation. Image Credit: Biotage
Biotage® ACT (Anti-Cross Talk) Plate Adapter (part number 414355SP) has been developed for mounting on top of the 96-well collection plate. The plate adapter “chimneys” lead the gas flow into the wells.
Evaporated gases are led away from surrounding sample wells, eddies are decreased, and the adapter minimizes the open surface area of the top of the wells, thus avoiding contamination of adjacent wells by the re-entry of the volatilized solvent containing the analyte. The plate adapter can be integrated into the present assays without modifying any other features of the technique.

Figure 10. Biotage® ACT Plate adapter in position on the 2-mL collection plate. Image Credit: Biotage

Figure 11. Evaporation on SPE Dry 96 using Biotage® ACT Plate Adapter. Image Credit: Biotage
The experimental design mentioned below was used to assess the impact of Biotage® ACT Plate Adapter on evaporation-related cross-talk: A 96-well plate’s single well (position C3) was spiked at a very high concentration with marker compound (amphetamine, 2 μg/mL) reflecting a high-level positive sample.
The adjacent wells were spiked at the technique LOQ. The amphetamine concentration in the adjacent wells was quantified with LC-MS/MS after evaporation with and without the Biotage® ACT plate adapter.
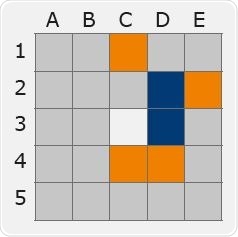
Figure 12a. Map of amphetamine concentration after evaporation with no plate adapter used. Image Credit: Biotage
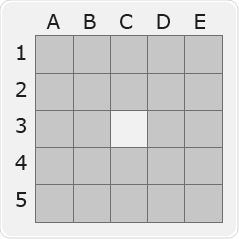
Figure 12b. Map of amphetamine concentration after evaporation using the plate adapter. Image Credit: Biotage
Key
 |
White (position C3) = high-level spike |
 |
Dark Blue = amphetamine concentration measured at >10 x LOQ reflecting gross cross contamination |
.jpg) |
Orange = amphetamine concentration measured at >2 x LOQ reflecting low level cross contamination |
.jpg) |
Grey = amphetamine concentration measured at LOQ reflecting no cross-contamination |
From the above-mentioned data, it can be observed that samples evaporated with Biotage® ACT Plate Adapter exhibit a total absence of cross talk. By contrast, samples evaporated without Biotage® ACT Plate Adapter exhibit false positive outcomes with concentrations greater than 10 times the LOQ.
Solvent volume optimization
When regular “square well” collection plates are used, some solvents (for example, methanol) tend to “creep” up the corners of the collection plate at the time of evaporation, thus possibly resulting in well-to-well contamination.
This is more probable when the volume of the solvent surpasses the suggested volume for that specific plate, or when excessive rates of evaporation gas flow are employed. Biotage suggests the following optimum solvent volumes for Biotage square well collection plates.
Table 1. Source: Biotage
| Nominal well volume |
Recommended maximum solvent volume |
| 1 mL |
0.75 mL |
| 2 mL |
1.5 mL |
Alternative design: Round well plates
The solvent creep effect can be avoided by using collection plates with a circular well cross-section (p/n 121-5213).
Devolatilization of volatile analytes
Cross talk can also be reduced by using a method that is usually employed to avoid low recovery of volatile analytes while carrying out evaporation procedures.
For instance, the addition of tartrate solution or HCl to certain analyte suites to enable the formation of less volatile salt can minimize the chances of cross talk. Instead, the addition of a keeper solvent (for example, glycol) to avoid complete evaporation of more volatile extraction solvents may also be useful.
Other aspects of evaporation to consider
- Users should make sure that the evaporating gas flow rate is not too much
- Users must verify that evaporator needles are not located too close to the surface of the solvent
Sample mixing
In general, samples and/or reconstitute samples are mixed following evaporation in 96-well collection plates before performing LC-MS/MS analysis. It is essential to optimize solvent fill volumes and mixing speeds according to the design of the collection plate.
Biotage recommends the maximum solvent volumes for Biotage collection plates as shown in the following table.
Table 2. Source: Biotage
| Nominal Well Volume / Design |
Recommended Maximum Solvent Volume |
| 1 mL square |
0.5 mL |
| 2 mL square |
1.5 mL |
| 2 mL round |
1.7 mL |
There are chances of cross-contamination if maximum solvent fill volumes are surpassed. This is depicted in Figures 13a to 13c, using several collection plate designs that contain a dye to track cross-contamination.

Figure 13a. 0.5 and 0.75 mL volumes mixed in a 1-mL/well nominal volume square collection plate (Vortex-Genie, setting 3) showing potential cross-contamination when maximum fill volume is exceeded. Image Credit: Biotage

Figure 13b. 1.5 and 1.75 mL volumes mixed in a 2-mL/well nominal volume square collection plate (Vortex-Genie, setting 3) showing potential cross-contamination when max fill volume is exceeded. Image Credit: Biotage

Figure 13c. 1.7 and 1.8 mL volumes mixed in a 2-mL/well nominal volume round collection plate (Vortex-Genie, setting 3) showing potential cross-contamination when max fill volume is exceeded. Image Credit: Biotage
References
- L. Marshall et al., The Effects of Plate Type on the Prevalence of Cross-well Contamination While Using Automated Solid Phase Extraction Instruments. Poster presented at Pittcon 2011.
About Biotage
Biotage offers solutions, knowledge, and experience in the areas of analytical chemistry, medicinal chemistry, peptide synthesis, separation, and purification. Customers include pharmaceutical, clinical and biotech companies, companies within the food industry, and leading academic and government institutes. The company is headquartered in Uppsala and has offices in the US, UK, China, S. Korea, India, and Japan. Biotage has approx. 460 employees and had sales of 1,101 MSEK in 2019. Biotage is listed on the NASDAQ Stockholm.
Aim
Biotage is a global Life Science company that develops innovative and effective solutions for separation within organic and analytical chemistry, as well as for industrial applications. We help shape the sustainable science of tomorrow and our future society for the benefit of humankind. Our mission is to help our customers to make the world more sustainable, healthier, and cleaner.
This is Biotage
Customers
The company has a strong customer base of industry and academic partners, which include the world’s top 20 pharmaceutical companies and prestigious academic and government institutes such as the US National Institutes of Health, the US Centers for Disease Control and Prevention, and the Karolinska Institute in Sweden.
Biotage products are used by public authorities, academic institutions, contract research, and contract manufacturing organizations, as well as the pharmaceutical and food industries. The Biotage products rationalize the workflow of customers and reduce their impact on the environment, for example by using a lower volume of solvents. Customers use Biotage products e.g. in their development of new medicines and to analyze samples from hospital patients, in forensic laboratories, or for the analysis of environmental and food samples. Biotage also offers products to remove undesired substances from, for example, pharmaceuticals during the manufacturing process.
Locations
Headquartered in Uppsala, Sweden, Biotage AB also has facilities in Lund, Sweden; Charlotte, NC, USA; San Jose, CA, USA; Salem, NH, USA; Cardiff, UK; Bundang, S. Korea; New Dehli, India; Tokyo and Osaka, Japan; and Shanghai, China.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.