Novel Psychoactive Substances (NPS) are known to be emerging substances that have been structurally altered from prevailing regulated substances. Synthetic cannabinoids are a sub-category of NPS.
Synthetic cannabinoids are structurally associated with tetrahydrocannabinol (THC)—that is, agonists of the CB1 and CB2 receptors. They are marketed under a broad range of names, like Spice, K2, and Legal Highs.1 Synthetic cannabinoids are easily available in convenience stores or found online as dried plant material or as liquids for e-cigarettes.
A total of 190 new synthetic cannabinoids were identified by the EMCDDA and these were announced in 2019 as part of the early warning system.1 Compounds like these have been specifically produced to circumvent the prevailing laws with regard to scheduling guidelines and drug control.
Such substances pose a major public health concern because of their unidentified potency and a broad range of side effects. The EMCDDA had reported symptoms, such as psychosis, mass poisoning, nausea, stroke, agitation, and even death.2
The development of a new high-pressure liquid chromatography/triple quad mass spectrometry technique enables the precise and efficient detection of 12 synthetic cannabinoids in whole blood through the LC-MS/MS method. All samples are fortified with internal standard, then buffered, and finally extracted using the Supported Liquid Extraction (SLE) technique.
Analytes
Analytes include ADMB FUBINACA, APP BINACA, 4-cyano CUMYL BINACA, 5-Fluoro CUMYL P7AICA, 4-Fluoro MDMB BINACA, MMB-FUBINACA, 5-Fluoro MDMB PICA, MMB-FUBINACA, ADMB CHMINACA, 5-Fluoro CUMYL PINACA, 5-Fluoro ADB/5-Fluoro-AEB, CUMYL PeGACLONE F, and MDMB-4en-PINACA.
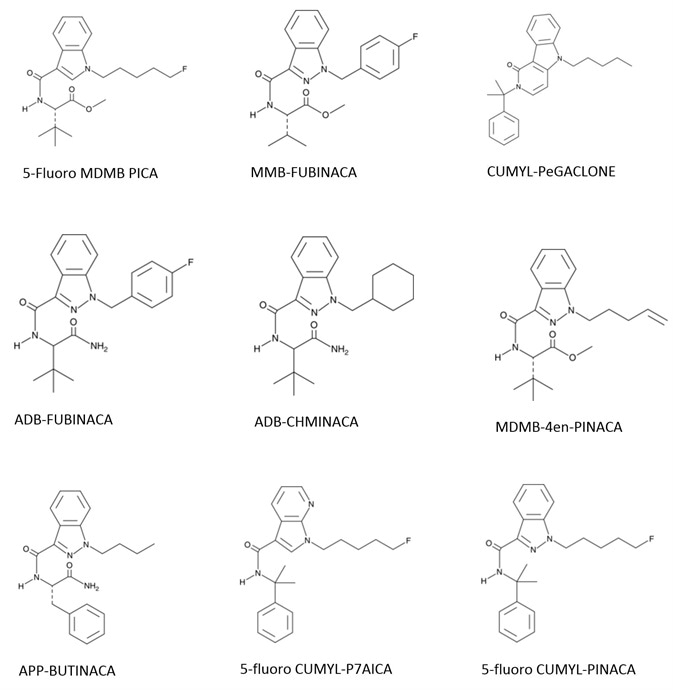
Figure 1. Synthetic Cannabinoid Structures. Image Credit: Biotage
Sample preparation procedure
Format
ISOLUTE® SLE+ 1 mL Supported Liquid Extraction Columns, Part Number 820-0140-CG.
Sample pre-treatment
First, samples of whole blood were fortified and then 500 μL of sample, controls, and calibrators were aliquoted into the extraction tubes. Using 25 μL of internal standard, all samples were fortified and then vortexed to mix. The samples were also buffered with 500 μL of deionized water and then vortexed.
Sample loading
The SLE+ column was loaded with 750 μL of buffered sample, making sure that a suitable collection tube is in place. Positive pressure of 2 to 5 psi was then applied using a Biotage PRESSURE+ 48 Positive Pressure Manifold to allow the sample to flow into the column. This sample was permitted to absorb for 5 minutes.
Analyte elution
Next, 2 mL of ethyl acetate is added to elute gravitationally. Through positive pressure, any surplus solvent is gently pushed. The process is repeated by adding 2 mL of ethyl acetate and allowing it to elute gravitationally. Through positive pressure, any remaining solvent is also pushed through.
Post extraction
Using a ramped flow technique, the extracts were evaporated with the help of a Biotage TurboVap® LV: 1.5 L/minute for 5 minutes and then 2.5 L/minutes until dryness. The temperature of the water bath was fixed at 30 °C.
Reconstitution
Samples were reconstituted by introducing 200 μL of 50:50 (v/v) methanol/deionized water with 1% formic acid.
HPLC conditions
Instrument
Waters UPLC® Acquity System Xevo TQS (Waters, Milford, MA, USA)
Column
Waters Acquity BEH C18 (100 mm x 2.1 mm, 1.7 µm)
Mobile phase
- A: high-purity deionized water with 0.1% formic acid
- B: high-purity methanol and acetonitrile (80:20)
Flow rate
0.4 mL/minute
Injection volume
10 μL
Column temperature
50 °C
Table 1. HPLC Gradient conditions. Source: Biotage
| Step |
Time (min.) |
Flow Rate (mL/min.) |
%A |
%B |
| 1 |
0 |
0.4 |
60 |
40 |
| 2 |
3 |
0.4 |
20 |
80 |
| 3 |
4 |
0.4 |
10 |
90 |
| 4 |
4.1 |
0.4 |
5 |
95 |
| 5 |
4.5 |
0.4 |
5 |
95 |
MS conditions
All experiments were performed on a UPLC® system (Waters Acquity System, Waters, and Manchester, United Kingdom) linked to a tandem quadrupole mass spectrometer (Xevo TQS, Waters, Milford, MA, United States).
MS/MS detection was carried out using electrospray ionization (ESI) operating in positive ion mode with multiple reaction monitoring (MRM). The source temperature was fixed at 150 °C, the capillary voltage at 1.0 kV, and the nitrogen desolvation gas was heated up to 400 °C with a flow rate of 800 L/hour.
Table 2. MS conditions and retention times for target analytes. Source: Biotage
| Analyte |
M+1 |
Collision |
RT |
| APP BINACA |
365.3 > 201.2
365.3 > 320.3 |
24
14 |
2.47 |
| ADMB FUBINACA |
383.1 > 253
383.1 > 109 |
24
46 |
2.48 |
| 5F CUMYL P7AICA |
368.2 > 250
368.2 > 119 |
14
32 |
2.65 |
| 4 cyano CUMYL BINACA |
361.1 > 226.1
361.1 > 243.1 |
22
10 |
2.73 |
| 5F MDMB PICA |
377.2 > 232.1
377.2 > 144.1 |
16
38 |
3.00 |
| 4F MDMB BINACA |
364.3 > 219.2
364.3 > 304.3 |
24
14 |
3.05 |
| ADMB CHMINACA |
371.1 > 326.1
371.1 > 241.1 |
16
26 |
3.12 |
| MMB-FUBINACA |
384.3 > 253.2
384.3 > 109.1 |
22
38 |
3.13 |
| 5F ADB/5F-AEB |
378.2 > 145.1
378.2 > 233.2 |
38
22 |
3.28 |
| 5F CUMYL PINACA |
368.1 > 233
368.1 > 213 |
18
28 |
3.40 |
| MDMB-4en-PINACA |
358.2 > 298.2
358.2 > 171.1 |
24
16 |
3.65 |
| CUMYL PeGACLONE |
373.2 > 255
373.2 > 119 |
10
26 |
3.79 |
Results and discussion
Analytical standards offered by Cayman Chemical (Ann Arbor, Michigan) were prepared in methanol and additionally diluted to the corresponding concentrations for controls and calibrators (see Table 3).
Table 3. Reporting limits for all respective compounds. Source: Biotage
| Analyte |
Reporting Limit (ng/mL) |
| APP BINACA |
0.1 |
| 4 cyano CUMYL BINACA |
0.1 |
| 5F MDMB PICA |
0.1 |
| 4F MDMB BINACA |
0.1 |
| ADMB CHMINACA |
0.1 |
| MMB-FUBINACA |
0.1 |
| 5F CUMYL PINACA |
0.1 |
| MDMB-4en-PINACA |
0.1 |
| 5F ADB/5F-AEB |
0.2 |
| 5F CUMYL P7AICA |
0.5 |
| CUMYL PeGACLONE |
0.5 |
| ADMB FUBINACA |
1.0 |
A bulk serum cut-off calibrator and negative and positive controls were then prepared by aliquoting 50 μL of the bulk serum material into 950 μL of whole blood.
Using a C18 column defined in Table 1, chromatographic separation was obtained across an elution gradient. The extracted ion chromatograms (see Figure 2) and the retention times observed for all of the analytes are shown in Table 2. The observed recovery was more than 60% for all compounds, as indicated in Figure 3.
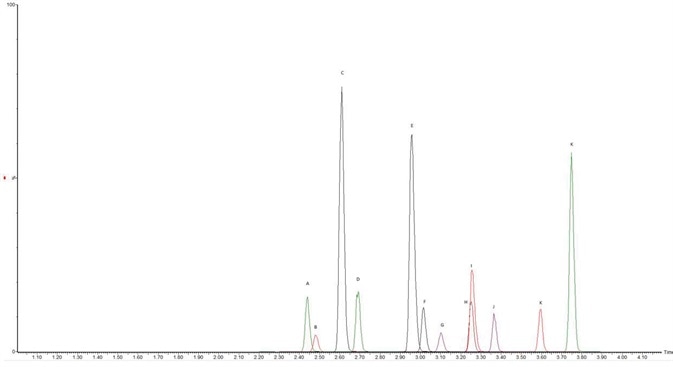
Figure 2. Method Chromatograph. (A) ADMB FUBINACA, (B) APP BINACA, (C) 5-Fluoro CUMYL P7AICA, (D) 4-cyano CUMYL BINACA, (E) 5-Fluoro MDMB PICA, (F) 4-Fluoro MDMB BINACA, (G) MMB-FUBINACA, (H) 5-Fluoro ADB/5-Fluoro EMB, (I) ADMB CHIMINACA, (J) 5-Fluoro CUMYL PINACA, (K) MDMB 4en PINACA, (L) CUMYL PeGACLONE. Image Credit: Biotage
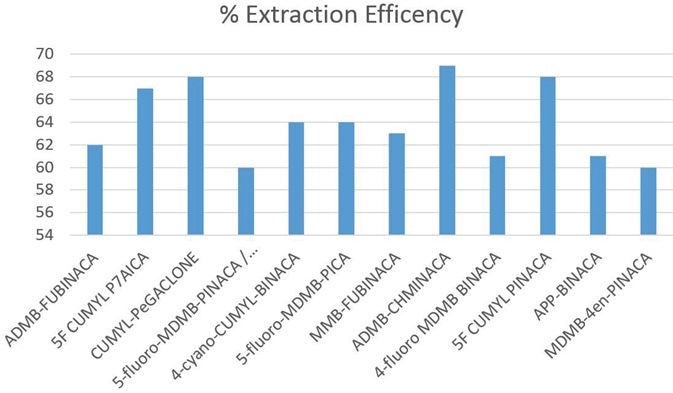
Figure 3. Recoveries for synthetic cannabinoids in blood at reporting limit using ISOLUTE® SLE+ 1 mL column. Image Credit: Biotage
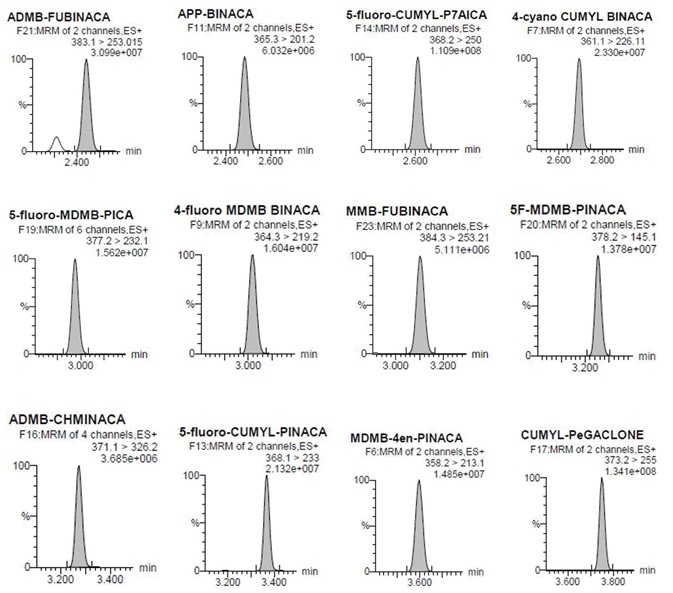
Figure 4. Individual chromatographs at the LOQ. Image Credit: Biotage
Conclusion
The ISOLUTE® SLE+ extraction technique with analysis by LC-MS/MS, described in this article, effectively isolated the novel synthetic cannabinoids from whole blood with more than 60% recoveries.
References
- EU Drug Markets Report (2019). Lisbon, Portugal: European Monitoring Centre for Drugs and Drug Addiction; https://www.emcdda.europa.eu/index_en. DOI: 10.2810/796253TD (Accessed 2/7/2020)
- PERSPECTIVES ON DRUGS: Synthetic cannabinoids in Europe (2019). Lisbon, Portugal: European Monitoring Centre for Drugs and Drug Addiction; https://www.emcdda.europa.eu/ (Accessed 2/7/2020)
About Biotage
Biotage offers solutions, knowledge, and experience in the areas of analytical chemistry, medicinal chemistry, peptide synthesis, separation and purification. Customers include pharmaceutical, clinical and biotech companies, companies within the food industry and leading academic and government institutes. The company is headquartered in Uppsala and has offices in the US, UK, China, S. Korea, India, and Japan. Biotage has approx. 460 employees and had sales of 1,101 MSEK in 2019. Biotage is listed on the NASDAQ Stockholm.
Aim
Biotage is a global Life Science company that develops innovative and effective solutions for separation within organic and analytical chemistry, as well as for industrial applications. We help shape the sustainable science of tomorrow and our future society for the benefit of humankind. Our mission is to help our customers to make the world more sustainable, healthier, and cleaner.
This is Biotage
Customers
The company has a strong customer base of industry and academic partners, which include the world’s top 20 pharmaceutical companies and prestigious academic and government institutes such as the US National Institutes of Health, the US Centers for Disease Control and Prevention and the Karolinska Institute in Sweden.
Biotage products are used by public authorities, academic institutions, contract research and contract manufacturing organizations, as well as the pharmaceutical and food industries. The Biotage products rationalize the workflow of customers and reduce their impact on the environment, for example by using a lower volume of solvents. Customers use Biotage products e.g. in their development of new medicines and to analyze samples from hospital patients, in forensic laboratories, or for the analysis of environmental and food samples. Biotage also offers products to remove undesired substances from, for example, pharmaceuticals during the manufacturing process.
Locations
Headquartered in Uppsala, Sweden, Biotage AB also has facilities in Lund, Sweden; Charlotte, NC, USA; San Jose, CA, USA; Salem, NH, USA; Cardiff, UK; Bundang, S. Korea; New Dehli, India; Tokyo and Osaka, Japan; and Shanghai, China.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.