This article explains a process in which Biotage® Lysera for matrix micropulverization is used for sample pre-treatment and isolation of a panel of 49 drugs of abuse from human hair before clean up using the ISOLUTE® SLE+ supported liquid extraction method.
The article involves procedures that are optimized for 400 µL capacity column format and also for 400 µL and 200 µL supported liquid extraction plate formats meant for higher throughput applications.
The technique delivers clean extracts and allows analyte recoveries typically higher than 80% with less than 10% RSDs for all analytes and LLOQ from 1 ng/mL.
The ISOLUTE® SLE+ Supported Liquid Extraction plates and columns provide an efficient option to conventional liquid-liquid extraction (LLE) method for bioanalytical sample preparation, enabling high analyte recoveries, considerably decreased preparation time, and emulsion formation.
Analytes
Methamphetamine, Amphetamine, MDA, MDEA, MDMA, BZE, Morphine, Hydromorphone, Oxymorphone, Oxycodone, Dihydrocodeine, Norfentanyl, Mephedrone, 7-amino-clonazepam, 7-amino-flunitrazepam, Codeine, Hydrocodone, Cocaine, 6-MAM, EDDP, Norketamine, Zopiclone, Zaleplon, Ketamine, Norbuprenorphine, Flunitrazepam, Nitrazepam, α-OH-triazolam, Clonazepam, Estazolam, Oxazepam, Zolpidem, Temazepam, Methadone, Alprazolam, Bromazepam, Lorazepam, 2-OH-ethylflurazepam, α-OH-alprazolam, Nordiazepam, Triazolam, Midazolam, Diazepam, Flurazepam, Fentanyl, LSD, PCP, and Buprenorphine.
Internal standards
Morphine-D3, Amphetamine-D5, 6-MAM-D3, BZE-D3, and Diazepam-D5.
Figure 1. Example structures by class. Image Credit: Biotage
Sample preparation procedure
Format
- ISOLUTE® SLE+ 200 µL capacity plates (p/n 820-0200-P01)
- ISOLUTE® SLE+ 400 µL capacity plates (p/n 820-0400-P01)
- ISOLUTE® SLE+ 400 µL capacity columns (p/n 820-0055-B)
Matrix preparation
First, 20 mg of hair is weighed into 2 mL Biotage® Lysera tubes consisting of 4 x 2.4 mm stainless steel beads. Then, 1 mL of methanol to every hair sample is spiked with ISTD (1 ng/mL).
Micropulverization procedure
Biotage® Lysera: 3 x 5.3 m/seconds for 3 minutes with a dwell time of 20 seconds. Tubes are centrifuged at 13300 rpm for 10 minutes.
Post micropulverization
Extracts are transferred and evaporated using a TurboVap® LV at 20 °C, and reconstituted in up to 400 µL (50:50, v/v) methanol:water.
Supported liquid extraction conditions
Table 1. Source: Biotage
| |
ISOLUTE SLE+ 200 µL Plate,
Part Number 820-0200-P01 |
ISOLUTE SLE+ 400 µL Plate,
Part Number 820-0400-P01 |
ISOLUTE SLE+ 400 µL Columns,
Part Number 820-0055-B |
| Sample loading |
Load 200 µL of reconstituted supernatant to ISOLUTE® SLE+ sorbent and apply a pulse of pressure 3–5 seconds to initiate flow. Allow the sample to absorb for 5 minutes. |
Load 400 µL of reconstituted supernatant to ISOLUTE®SLE+ sorbent and apply a pulse of pressure 3–5 seconds to initiate flow. Allow the sample to absorb for 5 minutes. |
Load 400 µL of reconstituted supernatant to ISOLUTE®SLE+ sorbent and apply a pulse of pressure 3–5 seconds to initiate flow. Allow the sample to absorb for 5 minutes. |
| Analyte Extraction |
Apply DCM/IPA (95/5, v /v, 300 µL) allow to flow under gravity for 5 minutes Apply a further aliquot of MTBE (300 µL) and allow to flow under gravity for 5 minutes. For complete removal apply a pulse of positive pressure at 10 psi (10–20 seconds). |
Apply DCM/IPA (95/5, v/v, 600 µL) allow to flow under gravity for 5 minutes. Apply a further aliquot of MTBE (600 µL) and allow to flow under gravity for 5 minutes. For complete removal apply a pulse of positive pressure at 10 psi (10–20 seconds). |
Apply DCM/IPA (95/5, v/v, 600 µL) allow to flow under gravity for 5 minutes. Apply a further aliquot of MTBE (600 µL) and allow to flow under gravity for 5 minutes. For complete removal apply a pulse of positive pressure at 10 psi (10–20 seconds). |
Post elution and reconstitution
Extracts are evaporated at 40 °C in the presence of 100 µL of 50 mM HCl in MeOH to prevent evaporative losses of amphetamines. For column and plate formats, the TurboVap® LV and the Biotage® SPE Dry-96 were used, respectively. The extracts are reconstituted using a combination of mobile phase A and mobile phase B (80/20, v/v, 200 µL).
UHPLC conditions
Instrument
Shimadzu Nexera UHPLC
Column
Restek Raptor™ Biphenyl 2.7 µm (100 x 2.1 mm)
Mobile phase
- A: 2 mM Ammonium Formate (aq) 0.1% formic acid
- B: 2 mM Ammonium Formate (MeOH) 0.1% formic acid
Flow rate
0.4 mL/minute
Injection volume
5 μL
Column temperature
30 °C
MS conditions
Instrument
Shimadzu 8060 Triple Quadrupole MS utilizing ES interface
Nebulizing gas flow
3 L/minute
Drying gas flow
3 L/minute
Heating gas flow
17 L/minute
Interface temperature
400 °C
DL temperature
250 °C
Heat block temperature
300 °C
CID gas flow
270 kPa
Table 2. UHPLC Gradient. Source: Biotage
| Time (min) |
%A |
%B |
| 0 |
80 |
20 |
| 2.00 |
80 |
20 |
| 7.50 |
40 |
60 |
| 11.25 |
40 |
60 |
| 12.75 |
0 |
100 |
| 13.50 |
0 |
100 |
| 13.51 |
80 |
20 |
| 15.00 |
80 |
20 |

Image Credit: Biotage
Table 3. MS conditions for target analytes in positive mode. Source: Biotage
| Analytes |
MRM Transition |
Collision Energy |
| Morphine-D3 |
289.0>201.1
289.0>152.1 |
-26.0
-50.0 |
| Morphine |
286.0>152.1
286.0>201.1 |
-50.0
-25.0 |
| Oxymorphone |
302.00>227.1
302.00>198.1 |
-30.0
-45.0 |
| Hydromorphone |
286.0>185.0
286.0>157.0 |
-30.0
-40.0 |
| Amphetamine-D5 |
141.0>93.0
141.0>124.15 |
-15.0
-20.0 |
| Amphetamine |
136>91.05
136>119.1 |
-15.0
-14.0 |
| Methamphetamine |
150.0>90.95
150>119.1 |
-20.0
-14.0 |
| MDA |
180>105
180>77 |
-20.0
-40.0 |
| Dihydrocodeine |
302>119.05
302>171 |
-35.0
-45.0 |
| Codeine |
300.0>215.1
300.0>165 |
-25.0
-40.0 |
| 6-MAM-D3 |
331.0>165.1
331.0>211.1 |
-40.0
-25.0 |
| 6-MAM |
328.0>165.1
328.0>211.1 |
-40.0
-25.0 |
| MDMA |
194.0>163.1
194.0>105.0 |
-15.0
-25.0 |
| Oxycodone |
316.2>241.2 |
-20.0 |
| Mephedrone |
178.00>145.05
178.00>144.00 |
-20.0
-30.0 |
| Hydrocodone |
300.0>199.05
300.0>171.1 |
-30.0
-40.0 |
| MDEA |
208>163.05
208>105.05 |
-15.0
-25.0 |
| Nor-Ketamine |
223.9>125
223.9>179.05 |
-20.0
-15.0 |
| Nor-Fentanyl |
233.0>84.05
233.0>56.05 |
-20.0
-26.0 |
| BZE-D3 |
293.00>171.05
293.00>77.00 |
-20.0
-50.0 |
| BZE |
289.90>168.05
289.90>105.00 |
-20.0
-30.0 |
| Ketamine |
237.90>125.00
237.90>207.05 |
-30.0
-14.0 |
| 7-Aminoclonazepam |
285.90>222.10
285.90>121.10 |
-25.0
-29.0 |
| Cocaine |
304.00>182.05
304.00>82.05 |
-20.0
-30.0 |
| Zopiclone |
388.90>245.05
388.90>217.00 |
-15.0
-35.0 |
| Norbuprenorphine |
414.00>101.25
414.00>187.20 |
-39.0
-38.0 |
| LSD |
323.50>208.10
323.50>223.25 |
-29.0
-23.0 |
| 7-Aminoflunitrazepam |
283.90>135.05
283.90>227.05 |
-30.0
-26.0 |
| Zolpidem |
308.00>235.10
308.00>263.10 |
-35.0
-25.0 |
| Buprenorphine |
468.10>396.25
468.10>414.30 |
-40.0
-35.0 |
| Fentanyl |
337.00>188.10
337.00>105.00 |
-20.0
-40.0 |
| Flurazepam |
388.00>315.00
388.00>288.00 |
-20.0
-26.0 |
| PCP |
244.00>91.05
244.00>159.15 |
-35.0
-14.0 |
| Midazolam |
325.90>249.10
325.90>223.00 |
-35.0
-40.0 |
| Bromazepam |
315.80>182.10
315.80>209.10 |
-31.0
-27.0 |
| EDDP |
278.00>234.00
278.00>234.00 |
-30.0
-45.0 |
| Lorazepam |
320.80>275.00
320.80>229.05 |
-22.0
-30.0 |
| Oxazepam |
320.80>229.05
286.90>104.20 |
-23.0
-35.0 |
| Nitrazepam |
286.90>104.20
281.90>180.10 |
-25.0
-35.0 |
| Clonazepam |
315.90>270.05
315.90>214.05 |
-25.0
-38.0 |
| a-OH-Triazolam |
358.90>331.10
358.90>239.05 |
-28.0
-44.0 |
| 2-OH-et-flurazepam |
332.90>211.10
332.90>109.00 |
-37.0
-27.0 |
| Methadrone |
310.50>265.10 |
-16.0 |
| a-OH-Alprazolam |
324.90>216.10
324.90>205.10 |
-39.0
-46.0 |
| Nordiazepam |
270.90>140.05
270.90>208.10 |
-26.0
-28.0 |
| Zaleplon |
305.90>236.15
305.90>264.20 |
-28.0
-22.0 |
| Flunitrazepam |
313.90>268.10
313.90>239.10 |
-25.0
-35.0 |
| Estazolam |
294.90>267.05
294.90>205.05 |
-20.0
-40.0 |
| Temazepam |
300.90>255.05
300.90>177.05 |
-20.0
-39.0 |
| Triazolam |
342.90>308.10
342.90>239.05 |
-27.0
-41.0 |
| Alprazolam |
308.90>281.00
308.90>205.05 |
-25.0
-40.0 |
| Diazepam-D5 |
289.90>193.05
289.90>258.00 |
-32
-7.0 |
| Diazepam |
285.10>193.05
285.10 |
-32.0
-27.0 |
Results
The straightforward sample preparation technique provides clean extracts and allows analyte recoveries typically higher than 80% with lower than 10% RSDs for all analytes (see Figure 2) and LLOQs from 1 ng/mL (see Table 4) for all ISOLUTE® SLE+ formats utilized.
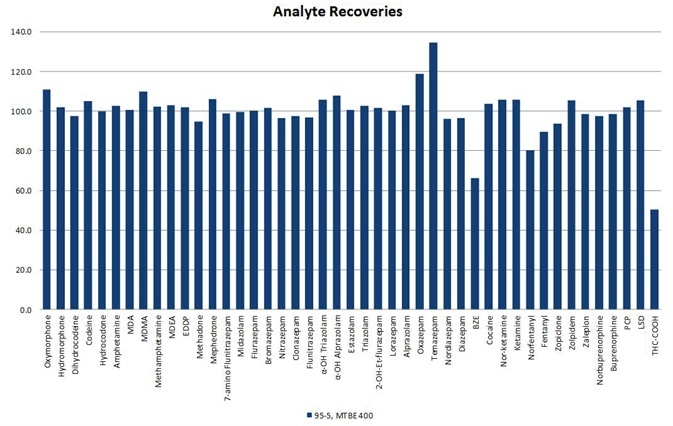
Figure 2. Representative analyte recoveries using the optimized SLE protocol for the 400-µL capacity column format (p/n 820-0055-B). Similar results were achieved using the 200-µL and 400-µL capacity plate formats. Image Credit: Biotage
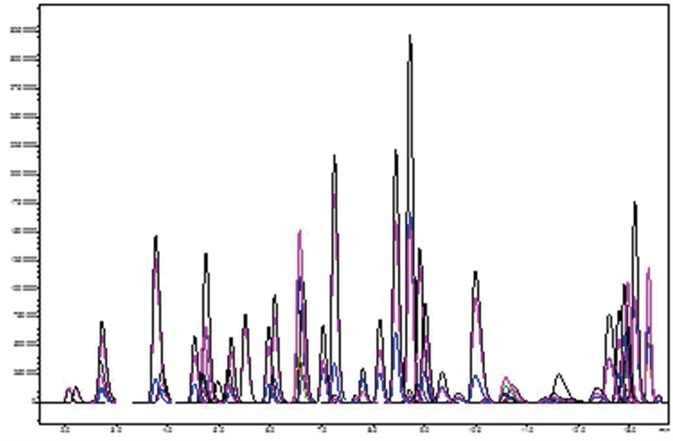
Figure 3. Representative chromatography for application analytes spiked at 1 ng/mL. Image Credit: Biotage
Calibration curve performance was examined from hair ((analytes (1to 1000 pg/mL) were introduced to methanolic NH4OH extraction solvent before micropulverization). Excellent linearity was observed for all analytes usually delivering greater than 0.99 r2 values.
Table 4 shows linearity performance and related LOQ for every analyte using the 400 µL-capacity column format, p/n 820-0055-B. The 200 µL and 400 µL capacity plate formats provided analogous results.
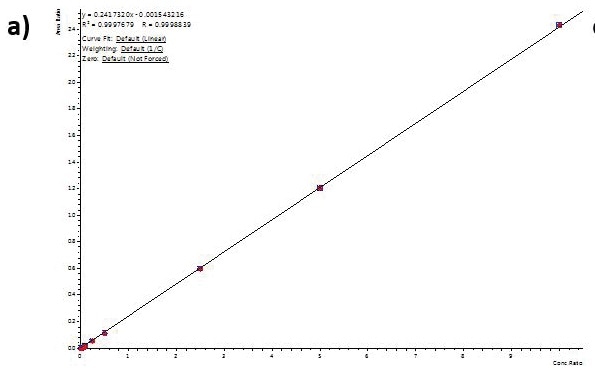
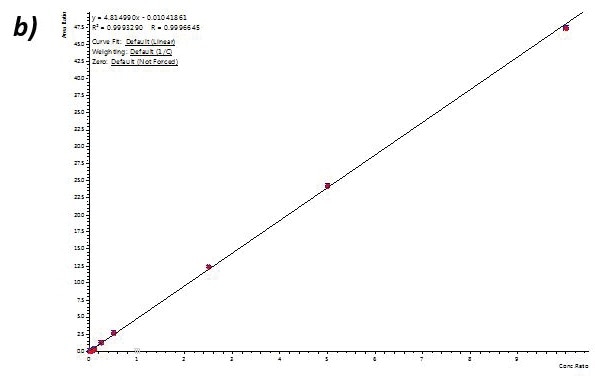
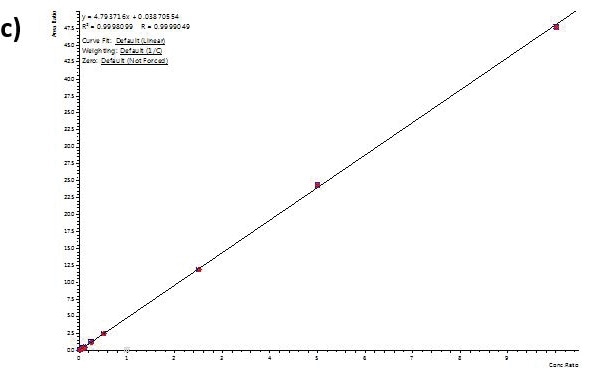
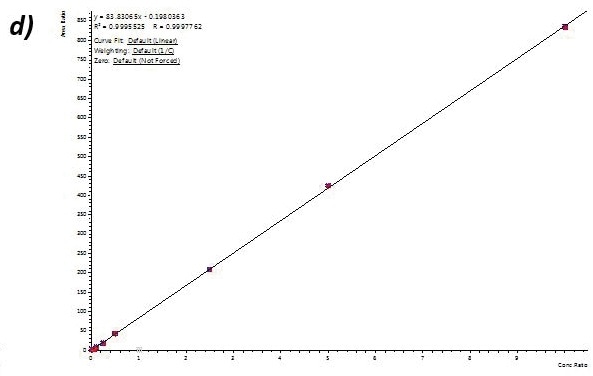
Figure 4. Calibration curves for Buprenorphine (a), 6-MAM (b), BZE (c), and Methamphetamine (d) extracted from human hair using the 400 µL capacity column format loading 400 µL of reconstituted extract. Similar results were achieved for the 200-µL and 400-µL capacity plate formats. Image Credit: Biotage
Table 4. Analyte calibration curve r2 and LOQ performance. Source: Biotage
| Analyte |
400 µL Load r2 |
400 µL Load LLOQ (pg/mL) |
| Morphine |
0.998 |
1 |
| Oxymorphone |
0.998 |
1 |
| Hydromorphone |
0.997 |
1 |
| Amphetamine |
0.998 |
1 |
| Methamphetamine |
0.998 |
1 |
| MDA |
0.998 |
10 |
| Dihydrocodeine |
0.998 |
<1 |
| Codeine |
0.998 |
1 |
| 6-MAM |
0.999 |
<1 |
| MDMA |
0.998 |
<1 |
| Oxycodone |
0.998 |
1 |
| Mephedrone |
0.996 |
1 |
| Hydrocodone |
0.998 |
1 |
| MDEA |
0.998 |
<1 |
| Nor-Ketamine |
0.996 |
1 |
| Nor-Fentanyl |
0.998 |
<1 |
| BZE |
0.999 |
<1 |
| Ketamine |
0.998 |
<1 |
| 7-Aminoclonazepam |
0.998 |
1 |
| Cocaine |
0.997 |
<1 |
| Zopiclone |
0.998 |
5 |
| Norbuprenorphine |
0.998 |
50 |
| LSD |
0.998 |
1 |
| 7-Aminoflunitrazepam |
0.999 |
1 |
| Zolpidem |
0.998 |
<1 |
| Buprenorphine |
0.996 |
1 |
| Fentanyl |
0.998 |
<1 |
| Flurazepam |
0.998 |
<1 |
| PCP |
0.998 |
1 |
| Midazolam |
0.997 |
1 |
| Bromazepam |
0.998 |
5 |
| EDDP |
0.999 |
<1 |
| Lorazepam |
0.998 |
10 |
| Oxazepam |
0.999 |
1 |
| Nitrazepam |
0.999 |
5 |
| Clonazepam |
0.998 |
5 |
| a-OH-Triazolam |
0.997 |
5 |
| 2-OH-et-flurazepam |
0.998 |
10 |
| Methadrone |
0.998 |
5 |
| a-OH-Alprazolam |
0.995 |
10 |
| Nordiazepam |
0.996 |
5 |
| Zaleplon |
0.997 |
1 |
| Flunitrazepam |
0.998 |
5 |
| Estazolam |
0.998 |
<1 |
| Temazepam |
0.998 |
5 |
| Triazolam |
0.998 |
1 |
| Alprazolam |
0.997 |
<1 |
| Diazepam |
0.997 |
1 |
About Biotage
Biotage offers solutions, knowledge, and experience in the areas of analytical chemistry, medicinal chemistry, peptide synthesis, separation, and purification. Customers include pharmaceutical, clinical and biotech companies, companies within the food industry, and leading academic and government institutes. The company is headquartered in Uppsala and has offices in the US, UK, China, S. Korea, India, and Japan. Biotage has approx. 460 employees and had sales of 1,101 MSEK in 2019. Biotage is listed on the NASDAQ Stockholm.
Aim
Biotage is a global Life Science company that develops innovative and effective solutions for separation within organic and analytical chemistry, as well as for industrial applications. We help shape the sustainable science of tomorrow and our future society for the benefit of humankind. Our mission is to help our customers to make the world more sustainable, healthier, and cleaner.
Customers
The company has a strong customer base of industry and academic partners, which include the world’s top 20 pharmaceutical companies and prestigious academic and government institutes such as the US National Institutes of Health, the US Centers for Disease Control and Prevention and the Karolinska Institute in Sweden.
Biotage products are used by public authorities, academic institutions, contract research and contract manufacturing organizations, as well as the pharmaceutical and food industries. The Biotage products rationalize the workflow of customers and reduce their impact on the environment, for example by using a lower volume of solvents. Customers use Biotage products e.g. in their development of new medicines and to analyze samples from hospital patients, in forensic laboratories, or for the analysis of environmental and food samples. Biotage also offers products to remove undesired substances from, for example, pharmaceuticals during the manufacturing process.
Locations
Headquartered in Uppsala, Sweden, Biotage AB also has facilities in Lund, Sweden; Charlotte, NC, USA; San Jose, CA, USA; Salem, NH, USA; Cardiff, UK; Bundang, S. Korea; New Dehli, India; Tokyo and Osaka, Japan; and Shanghai, China.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.