What are the benefits of microfluidic automation?
Microfluidic automation is the most straightforward available form of automation for endotoxin testing on the market today. It streamlines endotoxin testing in a platform that is very easy to set up, use and maintain. Microfluidic automation delivers high throughput, rapid assay setup, reduced hands-on time and simple training.
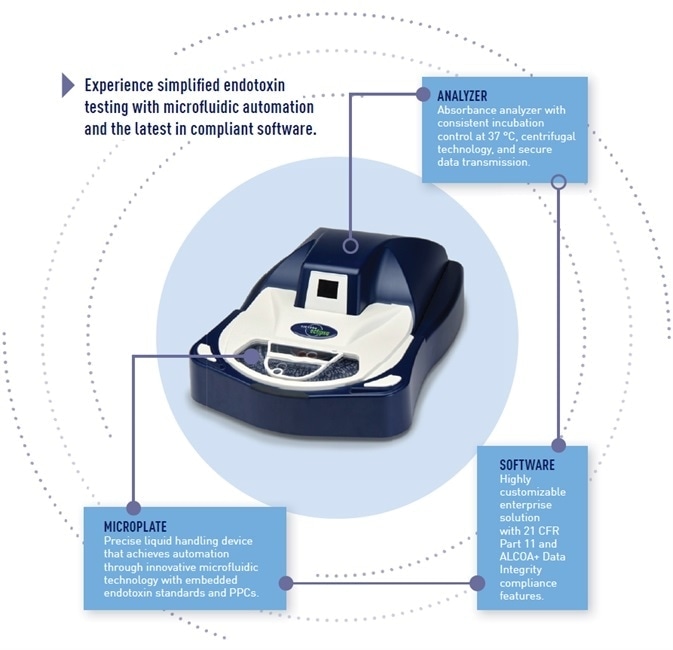
Image credit: Veolia Water Technologies & Solutions
The Sievers Eclipse Bacterial Endotoxins Testing (BET) Platform utilizes a traditional benchtop analyzer in respect of size, footprint and primary functionality that is coupled with the Eclipse microplate for automation of assay setup.
By providing microfluidic liquid handling and embedded endotoxin standards and positive product controls (PPCs) within the platform, quality control analysts have the ability to quickly begin a simple and fully compliant endotoxin assay in 9 minutes and in around 27 pipetting steps, with up to 21 samples.
Another advantage of microfluidic automation is associated with pipetting. In the endotoxin market, pipetting is one of the main contributors to errors and retests. Therefore, by lowering the pipetting steps under 30, the Eclipse platform reduces the risk of error that leads to expensive retests.
Additionally, the microfluidic liquid handling accurately measures all liquids for the end-user. This demonstrates the precision usually required in the physical action during the course of pipetting, which is eliminated through the exact design of the Eclipse microfluidic microplate.
Microfluidic automation allows labs to attain the high throughput and simple assay setup they desire, without the need to worry about footprint, compliance, or complex validations.
What components make up the Eclipse BET platform?
The Eclipse BET platform is made up of three components:
(1) An analyzer that is an incubating spectrophotometer, akin to other instruments employed for kinetic endotoxin testing;
(2) A microplate that automates the assay via microfluidic liquid handling, pre-deposited endotoxin standards and pre-deposited PPCs;
(3) Enterprise software that contains accessible protocols and libraries, customizable and flexible permissions and full compliance with 21 CFR Part 11 and data integrity standards.
How does microfluidic automation work?
Within the Eclipse microplate, microfluidic liquid handling allows for rapid, precise dispersion of reagents and samples with dramatically reduced volumes of sample and LAL.
This is accomplished utilizing metering chambers, microfluidic channels and centrifugal force as the microplate revolves to manage and automate all liquid measurement, flow and mixing when preparing for analysis.
Preloaded standards and PPCs are utilized to automate standard curves and PPC spikes. The closed microfluidic system inhibits environmental contamination and delivers an accurate 1:1 sample to lysate ratio.
With microfluidic automation, compendial endotoxin assays are conducted without difficulty and with a reduced number of retests.
Are standard curves automated?
For an endotoxin assay to maintain compliance, it is necessary that the end-user constructs at least a three-point standard curve in duplicate from a standard vial of regulated endotoxin; must have replicated negative controls; and must conduct each sample in duplicate with a PPC, also in duplicate.
However, due to the fact Eclipse automates steps with preloaded endotoxin standards covering up to a five-point standard curve and preloaded PPCs, the end-user only has to load Water for BET and samples onto the plate without any additional prep work.
The result is the capacity to assemble the assay in 9 minutes, in contrast to in excess of 60 minutes that other platforms necessitate. With the Eclipse platform, lab technicians are delighted with how straightforward and quick assay setup is.
Is it compliant?
Yes. The Eclipse platform utilizes FDA licensed LAL that is commercially available and meets all standards of the harmonized global pharmacopeia, USP <85>, EP 2.6.14 and JP 4.01.
In respect to data management and integrity, Eclipse software was developed with ALCOA+ principles as a leading principle to deliver a customizable enterprise solution with 21 CFR Part 11 and data integrity compliance features.
In short, the Eclipse platform includes:
- Analyst and lysate lot qualification in triplicate
- Compliance with 21 CFR Part 11 and data integrity guidelines
- Minimum three-point standard curve in duplicate utilizing standardized endotoxin
- Negative controls in duplicate
- Samples and PPCs in duplicate
- Use of FDA licensed LAL
How much LAL reagent is needed?
With just 1 mL LAL reagent, the Eclipse platform can run 21 samples. By reducing horseshoe crab (HSC) lysate use up to 90%, the Eclipse lowers demand on this precious natural resource and offers a BET assay that is fully compliant and one that is sustainable for the global HSC population.
Is training difficult?
As the Eclipse eliminates a majority of the complex assay setup, training and analyst certification are significantly more straightforward. Once the system has been completely validated, an analyst can be trained and certified in just one day utilizing the software’s template.
This function is convenient by allowing a lab manager to keep tabs on who is qualified, unqualified, or due for requalification.
Are there unique considerations around method transfer and validation?
Regardless of the present state of endotoxin testing, moving to an automated and efficient platform is surprisingly straightforward with the Eclipse platform, including method transfer, validation.
With expert guidance, the Sievers team supports customers with the implementation of the Eclipse, including suitability testing methods and product and system validation.
Additionally, the Eclipse platform naturally streamlines training programs, product validation, system validation and analyst and lysate qualifications. A complete IQ/OQ/PQ document is on hand, as are services offered by the Sievers team to advance and simplify the implementation of this pioneering endotoxin solution.
How are data review and sign-off handled with Eclipse?
In the current circumstances, it is vital that the data review process facilitates good security and efficiency. Quality control labs need to promptly review and sign off on data and batch release information – consistently in a secure manner – in order to deliver the product or in-process materials to keep up with the manufacturing process.
Thus, an enterprise software solution such as the Eclipse that facilitates secure access from any location is invaluable to life science businesses with several sites and/or remote workers. Eclipse software has supportive functionality for data review, including the capacity to set permissions for each user.
If a reviewer needs to distinguish between final product or in-process and raw materials, or even for water testing, the software offers the capacity to do that. Reports can also be observed for particular individual samples, if necessary.
All reports are tracked securely within the system and assay-specific audit trails in line with remaining compliant. As an enterprise solution, the Eclipse software facilitates straightforward, remote access for data review and electronic signature that makes it efficiently simple for the quality control lab to use.
From the quality manager and quality assurance professionals to analysts, all software users can take advantage of the flexible, secure options for reviewing and releasing samples.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.