What is zeta potential? When suspending particles in a liquid medium, the surface charges attract the counterions in the liquid and become attached to the particle surface, forming a firmly fixed inner Stern layer, and a protracted outer layer with a boundary of slipping plane.

Image Credit: Bettersize Instruments Ltd.
The ions and solvent in the shear layer travel in the medium as a unified whole when subjected to external force (i.e., electric field force). The potential defined at the slipping plane is known as the zeta potential.
The zeta potential particles in aqueous suspension are contingent on the chemical compositions on particles’ surfaces and also the surrounding environments such as pH, the concentration of the ions, and small-molecule additives.
Generally speaking, the concentration of the particles and the zeta potential are not directly related in a diluted aqueous system.
However, when the concentration of the particles exceeds a specific point, it is vital to consider the effect on zeta potential value as a result of the stronger particle-particle interactions and a greater number of charged ions in the solvent environment.
As a result, measuring and interpreting the zeta potential of highly concentrated samples have always been challenges. In this article, the zeta potentials of fat emulsion dispersed in water at different concentrations are measured by the BeNano 180 Zeta (Bettersize Instrument Ltd.).
Made up of soybean oil, glycerides, fatty acids, and phospholipids, fat emulsion is typically opaque with a milky-white color. The folded capillary cell compatible with the BeNano 180 Zeta has a light path as short as 4 mm, which allows for zeta potential measurement even for concentrated samples.
-1.jpg)
Figure 1. Fat emulsion at different concentrations. Image Credit: Bettersize Instruments Ltd.
Theory and instrumentation
The technology used to measure zeta potential is known as electrophoretic light scattering (ELS). In an ELS experiment, the sample is irradiated by a laser beam, where the scattered light is found at a forward angle of 12°.
The sample solution or suspension is exposed to an electric field applied to both ends of the sample cell, bringing about the electrophoretic movement of the charged particles in the sample.
As a consequence, the scattered light undergoes a frequency shift in contrast to the incident light because of the Doppler effect. The scattered light signals with a frequency shift are transformed into the phase shift using PALS analysis.
By the phase plot, the speed of electrophoretic movement per unit electric field, which is determined as the electrophoretic mobility µ, is acquired. Through Henry’s equation, the electrophoretic mobility µ and its zeta potential ζ can be related as follows:
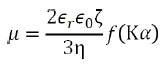
where ε0 represents the solvent dielectric constant in vacuum, εr indicates the relative dielectric constant, η stands for the solvent viscosity, f(Κα) is the Henry function, Κ signifies the reciprocal Debye length, α represents the particle radius, and Κα refers to the ratio between the thickness of the double layer and the particle radius.
For the zeta potential measurement, the BeNano 180 Zeta was used for this particular study. The sample is illuminated by a laser beam with a wavelength of 671 nm and a power of 50 m. An avalanche photodiode (APD) detector is assembled to collect scattered light signals.
By way of applying the PALS technique, the BeNano 180 Zeta effectively detects the zeta potential of samples even those with low electrophoretic mobility
Experiment
Since there was no trace of salt (which could potentially alter the zeta potential values by dissociating into cations and anions) found in the ingredients of fat emulsion, the distilled water was used to dilute the 20% w/v stock fat emulsion down to various concentrations.
The concentrations of the diluted fat emulsion suspensions range from 0.002% to 20% w/v. The temperatures of suspensions were determined by the built-in temperature control unit to be 25 °C.
The zeta potential measurements were conducted using the folded capillary cell. Each sample was measured at least three times to validate the repeatability of the results.
Results and discussion
The zeta potentials of fat emulsion at various concentrations are displayed in Figure 2 below. As exhibited in Figure 1, concentrated fat emulsion suspensions, whose concentrations range from 2% to 20%, had zeta potentials roughly around -5 to -7 mV.
For those suspensions whose concentrations were less than 2% but above 0.5% m/v, as concentrations decreased, the zeta potential’s absolute values increased.
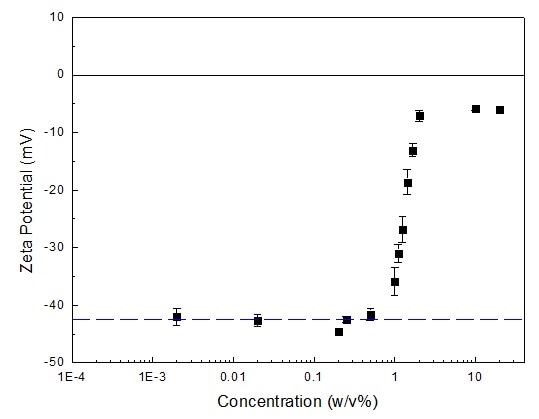
Figure 2. Zeta potential of fat emulsion at different concentrations. Image Credit: Bettersize Instruments Ltd.
For suspensions displaying concentrations ranging between 0.002% to 0.5% w/v, the zeta potentials were approximately -41 to -44 mV. Tiny fluctuations signify that in this concentration range the zeta potentials were independent of concentration.
Two potential reasons could account for the exceptionally low zeta potentials in highly concentrated fat emulsion suspensions.
The first one is relative to the particle-particle interaction. In concentrated suspensions, the particle-particle interaction was so strong that it suppresses the electrophoretic movements, and therefore results in a decrease in electrophoretic mobility of the particles, and consequently a decrease in the zeta potential.
The second reason is relative to the surface charges of lipid particles. When high enough particle concentrations are reached, the order in which the charged particles contribute to the ionic strength of the solvent environment can no longer be overlooked, leading to the decrease in the zeta potentials.
Conversely, as the concentrations decrease in the suspensions, the two aforementioned phenomena were less dominant, therefore generating the more accurate and stable zeta potential results.
Actually, where zeta potential concentrations are higher than 0.5% they were no longer the true zeta potential of the system but rather indicated the apparent zeta potential, which is not a true reflection of the actual potential value of the suspension system.
To ensure the true zeta potential value of an aqueous system is determined, it is crucial to use the appropriate dilutant, which maintains a steady environment the same as the stock solution; to dilute the stock solution to a suitable concentration range where the zeta potential is not dependent on the concentration.
Conclusions
The zeta potentials of fat emulsion suspensions at various concentrations were characterized successfully using the ELS technology of the BeNano 180 Zeta.
The results verify the capacities of the BeNano 180 Zeta when measuring the zeta potential of highly concentrated samples owing to its state-of-the-art optical system and the folded capillary cell with a short light path.
It can also be deduced that the true potential value of the system could not be reflected by the zeta potential results acquired from highly concentrated samples.
In order to acquire the true zeta potential results, a proper dilutant to dilute the concentrated sample to a suitable range should be used. For an unknown aqueous system, it is advised to conduct a concentration titration experiment to establish the optimal concentration range.
About Bettersize Instruments Ltd.

With over 25 years experience developing and manufacturing particle characterization instruments, Bettersize has introduced breakthrough technology in the field of particle size & shape measurement.
By achieving high quality and superior performance, our instruments provide precise analysis results of particle size, particle shape, and powder characteristics, helping scientists and engineers to understand material properties, facilitate research and improve production efficiency.
Bettersize product line for particle size and shape analysis includes instruments of all needs and budgets, from basic to advanced research models. These instruments are widely applied in Pharmaceuticals, Battery materials, Mining and minerals, Metals, Chemicals and Surface coatings, measuring materials with size ranges from nanometer to millimeter.
Focused on technology innovation, instruments manufacturing, application support and after-sales services, Bettersize provides expertise and professional solutions and assures customers the highest confidence in our products.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.