Sponsored Content by Bruker BioAFMReviewed by Mychealla RiceDec 22 2022
Critical factors that govern cell adhesion, morphogenesis, cell differentiation, and cancer invasion include the molecular connections between cells and their extracellular matrix surroundings, their 3D topography, and the associated mechanical characteristics.1-3
Atomic force microscopy (AFM) is a comprehensive multi-parametric imaging method that generates 3D profiles of the faces of molecules and cells in the nm range. It also allows for the evaluation of nanomechanical characteristics (adhesion, elasticity, etc.) and the imaging of structural changes occurring at the molecular level.
Tracing tissue specimens and larger multi-cellular layers, in addition to the nanomechanical evaluation of the extracellular matrix and its embedded cells, need a (semi)automated AFM method with millimeter precision in x,y with nm–resolution.
Significant differences in the topography of tissue samples, along with fluidity and stickiness of membrane and extracellular proteins, need more mobility in the z-axis of the AFM.3

Figure 1. JPK HybridStage integrated into a NanoWizard system on an inverted Nikon microscope. Image Credit: Bruker BioAFM

Figure 2. JPK HybridStage on a Zeiss upright AxioZoom V.16 fluorescence macroscope with an Andor EMCCD camera for force mapping experiments on non-transparent tissue samples. Image Credit: Bruker BioAFM
Technical solution
Bruker BioAFM has created a highly adaptable solution called HybridStage. It is outfitted with three scanner units, each of which may move the tip or the sample. A common NanoWizard AFM head has the xyz tip scanner with a scan range of 100 x 100 x 15 µm3.
The HybridStage has an xyz sample scanner with a configurable scan range of 100 x 100 x 100 µm3, 200 x 200 x 200 µm3, or 300 x 300 x 300 µm3, which can be adjusted independently depending on the individual application. It also has a motorized device for moving big samples (20 x 20 mm2).
The variety of scanners, along with optical tiling and multi-region AFM probing (figures 6, 7) allows for multiparametric characterization of soft materials across a vast area, as well as additional optical data sets on inverted and upright microscopes, respectively (figures 1, 2).
Single cell force spectroscopy and membrane tethers
The most common operations in single cell force spectroscopy (SCFS) are the investigation of the adhesion of individual cells on (bio)materials, the identification of extracellular matrix proteins, and the evaluation of cell-cell interactions.4
As a result of the influence of membrane tethering, the pulling length needed to remove cells from a substrate typically surpasses several tenths of microns.5
The versatile, modular architecture of the HybridStage, with 3D sample scanners that can scan in the range of 100 x 100 x 100 µm3, 200 x 200 x 200 µm3, or 300 x 300 x 300 µm3 can fulfill this need.
Figure 3 depicts the detaching curve of a fibronectin-functionalized cantilever from a Vero cell. After attaining the cell surface at a piezo height of about 60 µm, separation is achieved at a final piezo height of 105 µm.
The membrane tube structures, with force plateaus of 1-15 µm in length, are the primary cause for a total separation distance of roughly 45 µm in this case.
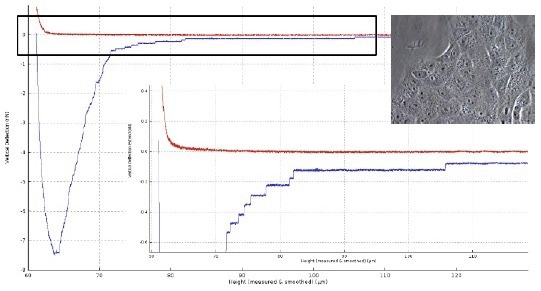
Figure 3. Force distance curve of a Vero cell (insert right: with an optical microscope image of Vero cells layer) in contact with a fibronectin-coated tipless cantilever (50 μg/ml for 30 min; TL-2 from NanoWorld). Force settings: 3D piezo scanner 100 x 100 x 100 μm3, 1 nN, z-speed 10 μm/s, contact 10 sec. The graph of the force curve (lower right) is the zoom of data in the black box. Image Credit: Bruker BioAFM
Optical focus control with JPK SlimFocus
The side-effect of a long-ranged z-movement for the tip and specimen scanner is that either the cantilever or the sample drifts out of the optical frame. As a result, the JPK SlimFocus (Figure 4) aligns the objective lens motion with the presently used z-scanner (figure 5).
The JPK SlimFocus control program allows users to modify the z-position with a slider manually, and define two marker locations, such as for the tip/sample. During AFM research, the optical focus motion may be synced with the z-scanner presently being used up to 150 µm.

Figure 4. JPK SlimFocus unit equipped with an objective lens mounted on a microscope turret. The sample stage is above the lens. Image Credit: Bruker BioAFM
Figure 5. SlimFocus software takes into account the height corrections needed if the objective lens and sample are surrounded by different media (liquid/air)6. Image Credit: Bruker BioAFM
Optical tiling and multi-region imaging
If the x,y piezo scan range and the optical observation frame (typically 100 µm) are substantially less than the sample size (in the range of mm) to be probed with the AFM, a problem occurs.
To widen the microscopic visual perspective, the motorized sample scanner performs a repeated pattern, obtaining the appropriate optical pictures of the sample, which are subsequently tiled and shown in the SPM application (Figure 6).
When tiling begins, the file picker generates an automatically labeled sub-folder. The calibrated file and the image file are saved in the sub-folder where they are conveniently available for data acquisition.
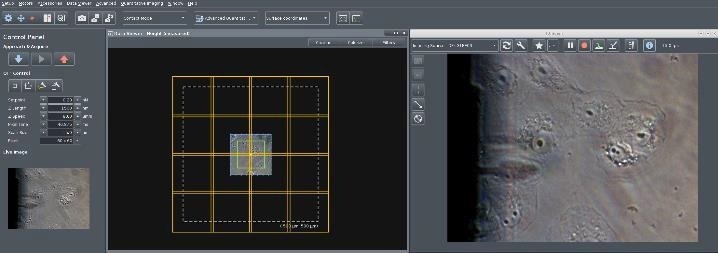
Figure 6. Screenshot of the NanoWizard control software. Right: Live camera image with Vero cells in liquid on an inverted microscope with optical phase contrast. The shadow of the retracted cantilever is visible. Left: The first optical image is calibrated in x,y with piezo accuracy using the AFM scanner (scan range marked in green, here 100 x 100 μm2). A “Tiling Tool” button allows the user draw a tiling rectangle on the surface. The software automatically considers the number of optical images required after selecting the tiling area (here 4 x4 images for 500 x 500 μm2). Image Credit: Bruker BioAFM
For multi-region AFM imaging, the user will be asked if they want to analyze more than one measuring location. They can then pick places of interest before the commencement of a measurement, which will then be shown in the information viewer with tiled optical images.
When the measurement starts, the points are handled consecutively. There are three measuring definitions: single points, lines, and rectangular regions.
The multi-scan option is available for several measuring modalities, including force mapping and spectroscopy, AFM imaging, QITM, and multiple DirectOverlay snapshots. An example of multiscan QI imaging of human Vero using an inverted optical microscope can be observed in Figure 7.
First, DirectOverlay was used to link the cantilever tip position with the associated optical image. Next, optical tiling with 4 x 4 pictures on 500 x 500 µm2 was carried out (see figure 6) to expand the area of focus. Finally, a multi-scan range was chosen.
In this case, 4 x 4 QI scans, with separate map sizes of 50 µm, were carried out (frames are labeled in magenta). Figure 7 shows that 13 of the 16 maps have already been performed.
The green frame on position 14 shows the present map position. Using the “advanced force oscilloscope”, various data receivers can be engaged, such as height (of the sample) at force setpoint, adhesion (between cantilever and sample), slope (as an indication of sample stiffness) and reference force height(s) at a pre-determined value of the force setpoint.
In the example, depicted in Figure 7, two separate data viewer channels are presented (height at 100% of the setpoint and reference height at 80% of the setpoint). To improve the contrast of cellular components, the reference height is shown as a pixel difference picture.
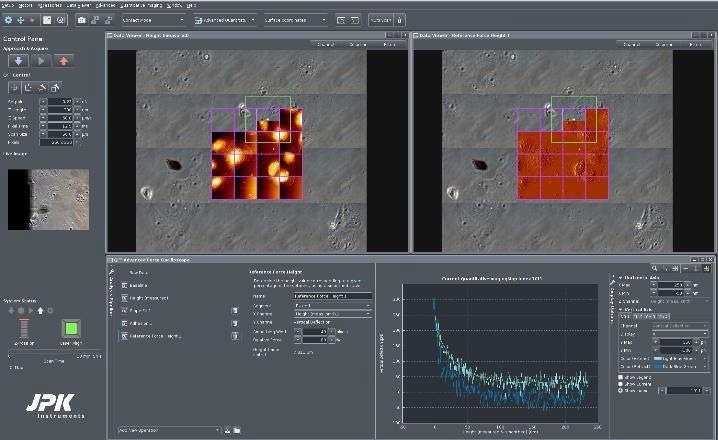
Figure 7. Screenshot of the NanoWizard HybridStage control software. Adherent Vero cells in phase contrast optical microscope, imaged in PBS buffer, under 37 °C temperature control in a JPK PetriDishHeater. Top: Optical tiling over 500 x 500 μm2 consists of 4 x 4 optical images. The selected multi-scan region is indicated by the magenta-colored frame: 4 x 4 maps with 50 x 50 μm2 size. Top left: Height channel maps. Top right: Pixel difference map of the same region. Bottom: Advanced force oscilloscope and a force curve for a specific index position. QI mode settings: Force 0.23 nN, z- length 300 nm, 256 x 256 pixels. A PFQNM-LC-A cantilever with a tip height of approx. 19 μm and blunt tip (radius 70 nm) was used. Image Credit: Bruker BioAFM
Multi-scan tissue mapping
To gain an improved understanding of the formation and progression of cancer, assessing modifications in the mechanical characteristics of cells and their surrounding extracellular matrix material seems to be critical.3
Employing the example of human cervix tissue, the advantages of the HybridStage and its method, in conjunction with an upright microscope, are shown here.
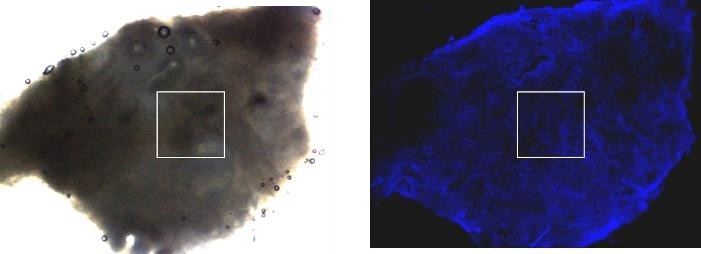
Figure 8. Zeiss upright AxioZoom V.16 (lens 0.5x) fluorescence macroscope was used for the overview transmission light image (left) and fluorescence image (right) of human cervix tumor tissue. Fluorescence staining was performed with HOECHST for the nucleus labeling. The white frames illustrate the 1000 x 1000 μm2 AFM mapping area. The optical images were done without an AFM head to obtain better optical quality for the fluorescence image. Image Credit: Bruker BioAFM
Owing to its extended working distance, the upright microscope enables the capture of optical images with or without the AFM head. Moreover, replacing the AFM head does not need a calibration of the sample.
The exceptional replacement precision of the head with an attached cantilever is in the range of < 2 µm in x,y,z. Once the area of interest has been identified in the optical image, it can be utilized to position the AFM probe concerning the region of interest (Figure 8).
A HybridStage with a 3D piezo sample scanner of 300 x 300 x 300 µm3 in combination with the mechanized staging was utilized to produce a multi-scan map of 1000 x 1000 µm2 with 5 x 5 single maps of 200 x 200 µm2 and a pixel distance of 10 µm apiece (Figure 9).
All individual mappings were evaluated utilizing the batch processing mode for indentation studies in the JPK data processing (DP) software. Conversely, the data can be exported, examined, and assembled using third-party software such as Matlab.
Figure 10 describes the frequency distribution of the apparent Young’s modulus of the multi-scan observed in Figure 9.
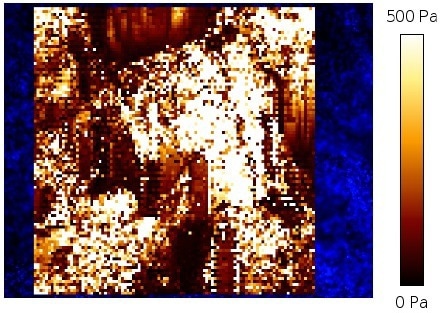
Figure 9. Overview of apparent Young’s modulus multi-scan map for 1000 x 1000 μm2 (compare figure 8) with 5 x5 individual force maps of 200 x 200 μm2. The single force maps were analyzed using the NanoWizard DP software. The indentation experiments were performed at 3 nN setpoint force with a Nanoworld-Cont cantilever with a 6 μm bead which was separately glued at the tip apex. Image Credit: Bruker BioAFM
When conducting a multi-scan force map, it may damage the cantilever, and the AFM measurements may result in unusable data across a greater region on a highly rough sample with changing heights.
Moreover, in particular soft specimens with adhesive surfaces, the cantilever barely breaks from the sample surface after a few tenths of micrometers, resulting in visible damage to the sample or loss of the membrane tube.
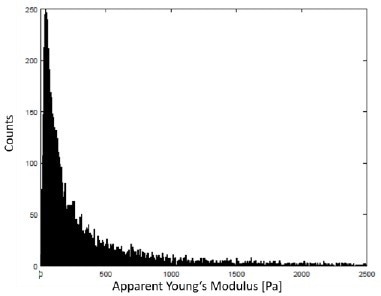
Figure 10. Histogram of the apparent Young’s modulus of the data displayed in Fig. 9 with a unimodal frequency distribution. A typical indentation depth at 3 nN setpoint force was around 1 μm – 3 μm. Image Credit: Bruker BioAFM
In this case, the z-range must be raised to successfully finish the test. To overcome this technological challenge, the NanoWizard software allows you to adjust the height of both the piezo-retract and the motor-retract.
In this manner, an individual mapping and complete scan can be optimized. Figure 11 illustrates that the tissue sample under consideration is quite rough and has a wide range of heights.
Even on the individual map of 200 x 200 µm2, the height difference is around 80 µm. However, the multi-scan is able to stably measure without stopping.
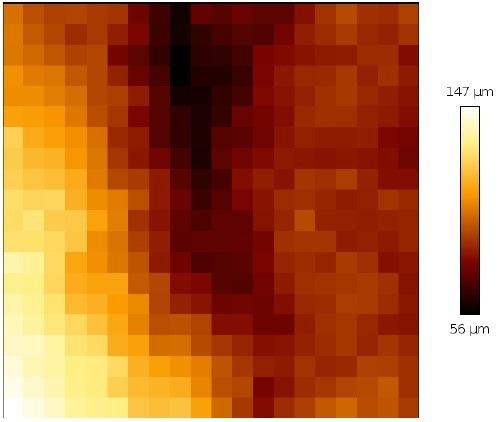
Figure 11. Height profile of a single map of 200 x 200 μm2. of the cervix tissue (map from a top right corner of the multi-scan area). Image Credit: Bruker BioAFM
HybridStage compatibilities
Optical microscope: Upright
- Zeiss Axio Zoom.V16
- Leica MF205
- Olympus MVX 10 with epi-fluorescence and brightfield
Optical microscope: Inverted
- Zeiss AxioObserver
- Nikon TE, Ti and Ti2 lines
- Leica DMI 6000/DMi8
- Olympus IX71 with epi-fluorescence and brightfield
Conclusion
The HybridStage is a flexible instrument for sophisticated, multiparametric AFM characterization of materials with mm to nm and pN resolution, which includes three scanner units that can move the tip/sample.
A variety of samples can be researched, including biomaterials, cells and micro aggregates, embryos and tissues, model organisms in developmental biology (zebrafish, C. elegans), and implants.
The enhanced z-piezo range, paired with wide-ranged motorized sample motion, is ideal for the nanomechanical evaluation of tissues used in cancer, nanomedicine, or developmental biology.
The HybridStage liberates studies from the limitations imposed by the AFM piezo range. Large-range tiling of optical images gives a clear visual perspective, enabling a quick setup of optically directed investigations and direct selection of the optical characteristics for examination.
Explore the sample, compile a list of fascinating properties for multi-scan, or even map force responses across very long scan ranges.
The HybridStage is a flexible, piezo-based sample scanner stage coupled with motorized XY sample mobility that allows for immediate access to everything visible.
The very adaptable answer for all sampling needs.
Sample courtesy
The data for tissue characterization shown in Figures 2 and 8 - 11 are kindly provided by Prof. J. Käs and Dr. Th. Fuhs (University Leipzig). The Vero cell samples were provided by Prof. A. Hermann and his group (Humboldt University Berlin).
References and Further Reading
- R. Pearce, G. J. Vansco, Macromolecules, 30: 5853 (1997)
- T.J. McMaster, J.K. Hobbs, P.J. Barham, M.J. Miles, Probe Microsc., 1: 43 (1997)
- J.K. Hobbs, T.J. McMaster, M.J. Miles, P.J. Barham, Polymer, 39: 2437 (1998)
- K.K. Jain, Drug Delivery Systems, Springer, 2008
- V.H. Mareau, R.E. Prud’homme., Macromolecules, 38: 398 (2007)
- V. Speranza, A. Sorrentino, F. De Santis, R. Pantani, Hindawi Publishing Corporation- The Scientific World Journal, (2014), Article ID 720157, http://dx.doi.org/10.1155/2014/720157
- sample courtesy Nic Mullin, University of Sheffield, UK
- https://www.youtube.com/user/JPKInstruments
- E. Zhuravlev, PhD thesis, Universtiy of Rostock, Germany, http://www.polymerphysik.unirostock.de/publication/theses/pdf/Zhuravlev.pdf
About Bruker BioAFM
Bruker BioAFM, former JPK Instruments AG, is a leading manufacturer of nano-analytical instruments - particularly based on atomic force microscope (AFM) and optical tweezers systems - for life sciences and soft matter applications.
We combine the highest technical skills with visionary applications. Our work applies nanotechnology in ways to provide solutions to challenges facing researchers in life sciences and soft matter today. Driven by inspiration and ambition, it is our conviction that only the best tools are good enough for the research of life. We are listening with the ear of a scientist in detail to the current challenges of our customers and find individual solutions for individual problems. This is how we understand our business.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.