FlowCam utilizes Flow Imaging Microscopy (FIM) to characterize particles in liquid biopharmaceutical formulations. When considering incorporating FIM into the workflow, certain key features of FIM instruments should be taken into account.

Image Credit: ShutterStock/Gorodenkoff
1. Sample Volume
The process of developing biopharmaceutical drugs incurs significant expenses, and the supply of biological ingredients is often limited. When it comes to effectively managing long-term costs, it is helpful to be able to detect any particle-based problems early in the process by analyzing the smallest volume sample of the formulation possible.
Determining the minimum sample volume required by a particle analyzer becomes essential to achieve optimal particle analysis without exhausting the sample.
2. Flexibility
Formulations in the pharmaceutical industry can differ significantly, leading to variations in particle characteristics. Hence, selecting a system that allows users to adjust instrument settings is vital during particle analysis.
For instance, drug formulations often contain stabilizing agent particles that influence the sample solution's refractive index. Providing the option to modify capture settings to suit specific formulations is essential for achieving accurate particle detection and characterization.
Likewise, when a broad range of particle sizes is expected, the FIM instrument should support multiple magnification objectives.
The FlowCam instrument, which offers interchangeable 2X, 4X, 10X, and 20X magnification objective lenses, and the capability to utilize various sizes of microfluidic flow cells, is the most desirable choice.
3. Instrument Sensitivity
Biopharmaceutical drugs consist of complex proteins and nucleic acids derived from living cells or organisms. Detecting these small ingredients, along with the corresponding stabilizing agents in drug formulations, can be challenging due to their refractive indices closely matching that of the suspending solution.
Therefore, having a sensitive instrument for particle analysis is crucial. The FIM (Image-based Analytical Method) defines particle properties using image segmentation threshold parameters.
The sensitivity of an FIM system lies in its capability to distinguish particle boundaries from the background in camera images. The option to customize image segmentation thresholding parameters becomes particularly vital when dealing with formulations containing high particle concentrations or significant amounts of stabilizers.
Some FIM systems use simple, dark pixel-only thresholding segmentation, which can lead to images of larger transparent or translucent aggregates being fractionated into smaller individual particle images or going undetected altogether – resulting in inaccurate particle size measurements, counts or concentration calculations.
The ability to demonstrate that particle counts and sizes in a formulation meet the USP standards plays a central role in FDA drug approval.1

A FlowCam collage of particles present in a biotherapeutic sample, including protein and silicone oil droplet aggregates. Image Credit: Yokogawa Fluid Imaging Technologies, Inc
To achieve better counts and characterization of translucent, biologically-derived particles such as protein aggregates, nucleic acids, and stabilizer ingredients commonly found in biopharmaceuticals, an instrument capable of setting threshold conditions on darker pixels, lighter pixels, or both relative to the background is more sensitive and effective.
4. Image Quality
Image quality is paramount for a flow imaging microscopy (FIM) system, as it directly influences the performance of the FIM instrument's software in pattern matching and particle classification.
The better the image quality, the more effectively the software can recognize patterns, classify particles, and provide accurate information on biological drug products' particle characteristics.
The true value of FIM lies in its ability to directly calculate physical properties and differentiate morphological features of subvisible particles from highly-resolved digital images.
FlowCam, a representative FIM instrument, utilizes fixed optics with known magnifications, enabling distance measurements on the image to be converted into real measurements of the object.
Unlike other particle imaging methods, such as light obscuration, laser diffraction, and dynamic light scattering, this approach avoids assumptions and relies on individual particle images for precise sample property calculations.
The accuracy and precision of the resulting data heavily depend on image quality.
Biopharmaceutical formulations often comprise translucent or similarly refractive particles, like proteins, in their suspension environment. Poor image quality may lead to inaccuracies in low-concentration calculations and increased measurement variations with low statistical confidence.
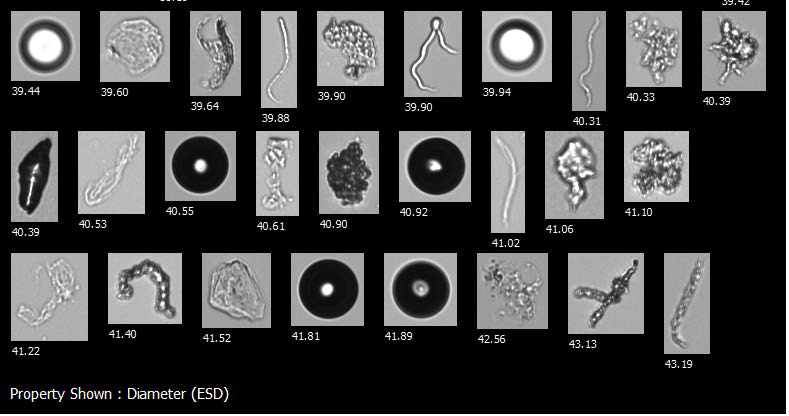
A FlowCam collage showing air bubbles, silicone oil droplets, protein aggregates, and other potential foreign contaminants in a biotherapeutic sample. Image Credit: Yokogawa Fluid Imaging Technologies, Inc
Poorly resolved images can make distinguishing protein aggregates and other sample contaminants difficult. Additionally, not being able to identify morphological differences among aggregated proteins – protein globules versus clusters of rod-shaped particles, for example – could result in bacterial contamination being missed in a sample.
In the context of FIM systems, prioritizing those with the highest image quality and sophisticated software capable of extracting accurate particle data becomes crucial when it comes to meeting stringent regulatory standards.
5. Ease of Analysis and Data Processing
Once data and images have been captured, it is important to have an easy way to analyze them. Opting for an FIM system with user-friendly software that enables efficient interaction with data and quick information extraction becomes a vital consideration.
Essential software features include:
- The ability to sort and filter particle images based on user-supplied criteria, providing immediate visual feedback.
- Sophisticated pattern recognition capabilities that instantly identify and display similar-type particles in a heterogeneous sample.
- The ability to create user-defined particle-type libraries, enabling instant enumeration of concentrations for specific particle types.
- Satellite software for post-processing data remotely or sharing data with others.
- A unified system for data acquisition and analysis, ensuring tight integration and eliminating lag time and data inconsistencies.
- Export functionality to Excel and other databases, offering added flexibility and enabling additional analysis.
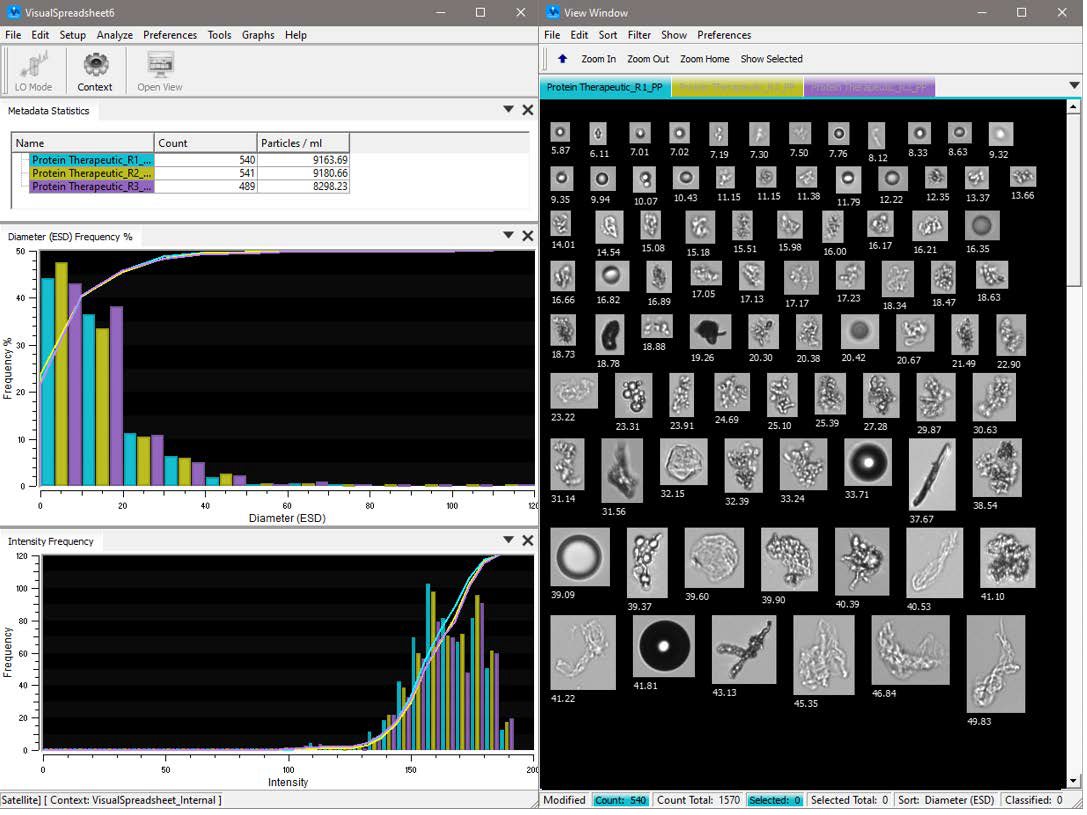
An example of the flexible user interface for FlowCam’s integrated software, VisualSpreadsheet®. Image Credit: Yokogawa Fluid Imaging Technologies, Inc
References
- United States Pharmacopeia. USP Subvisible Particulate Matter in Therapeutic Protein Injections.

This information has been sourced, reviewed and adapted from materials provided by Yokogawa Fluid Imaging Technologies, Inc..
For more information on this source, please visit Yokogawa Fluid Imaging Technologies, Inc.