A Contamination Control Strategy (CCS) identifies and evaluates contamination risks, explores mitigation options, and sets out plans for corrective and preventive actions. Its implementation addresses various contamination sources to improve sterility control.
Executing a strong CCS, however, requires the backing of experts in Manufacturing, Regulatory, Quality Control, Quality Assurance, and supportive departments.
This is a CCS as defined in Annex 1:
Contamination Control Strategy (CCS) – A planned set of controls for microorganisms, pyrogens, and particulates, derived from current product and process understanding that assures process performance and product quality. The controls can include parameters and attributes related to the active substance, excipient and drug product materials and components, facility and equipment operating conditions, in-process controls, finished product specifications, and the associated methods and frequency of monitoring and control.
Annex 1 (2022)
Background
Developing a comprehensive CCS focuses on optimizing environmental control and detection.
To ensure reliability and guide the creation of the CCS, manufacturers should incorporate Quality Risk Management (QRM) and Quality by Design into their production facility, equipment, personnel flows, and supporting processes.
It is also vital to examine past data and trends to properly gauge gaps, potential risks, and opportunities for mitigation; all of these contribute to forming a CCS. The design, procedures, organization, and technology controls are most effective when they holistically complement each other.
Development of a contamination control strategy
The proposed elements to be considered for the CCS are listed in Annex 1:
2.5 The development of the CCS requires detailed technical and process knowledge. Potential sources of contamination are attributable to microbial and cellular debris (e.g. pyrogen, endotoxin) as well as particulate (e.g. glass and other visible and sub-visible particles).
Elements to be considered within a CCS should include (but are not limited to):
- Design of both the plant and processes including the associated documentation.
- Premises and equipment.
- Personnel
- Utilities.
- Raw material controls – including in-process controls.
- Product containers and closures.
- Vendor approval – such as key component suppliers, sterilization of components and single-use systems (SUS), and critical service providers.
- Management of outsourced activities and availability/transfer of critical information between parties, e.g. contract sterilization services.
- Process risk management.
- Process validation.
- Validation of sterilization processes.
- Preventative maintenance – maintaining equipment, utilities, and premises (planned and unplanned maintenance) to a standard that will ensure there is no additional risk of contamination.
- Cleaning and disinfection.
- Monitoring systems - including an assessment of the feasibility of the introduction of scientifically sound, alternative methods that optimize the detection of environmental contamination.
- Prevention mechanisms – trend analysis, detailed investigation, root cause determination, corrective and preventive actions (CAPA), and the need for comprehensive investigational tools.
- Continuous improvement based on information derived from the above.
Annex 1 (2022)
Manufacturers can interpret the definition of a CCS, as outlined in Annex 1, in two ways: as a method for implementation or as an approach to demonstrate its effectiveness.
In either case, there is a three-step process for evaluating, approving, and rectifying the strategy. This process operates in a continuous cycle, where the outcome of one step feeds into the next in a loop.
There is not a single fixed starting point for all applications; the state of the manufacturing process determines where to commence or assess CCS development.
In the case of a new facility, a manufacturer may begin with the Assessment stage. At this juncture, the primary focus lies on identifying potential risks and gaps and collecting the necessary documentation and instruments to support a comprehensive CCS review.
For an established production or filling line, subject matter experts may initiate the Acceptance stage. Here, they can easily compile historical data and trends and gather evidence demonstrating the presence of an effective CCS.
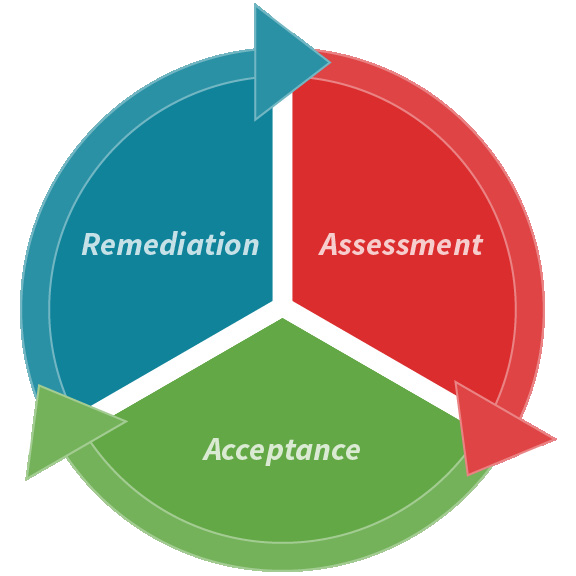
Figure 1. CCS development stages. Image Credit: Particle Measuring Systems
The three steps of developing a CCS: Assessment, acceptance, and remediation
Assessment: Outline the CCS
The establishment of a CCS should be built on a solid understanding of the need to maintain environmental control in sterile manufacturing.
It is crucial to examine and reference production principles, scientific basics, regulations, codes, accepted standards, and the latest innovations to ensure CCS development compliance. The most effective way to conduct a comprehensive assessment is by applying the essential principles of QRM.
Beginning with the preparation of the Risk Assessment, it is vital to comprehend any changes or additions to the CCS. ICH Guidance Q9 (Figure 2) recommends that experts in the field question potential risks or gaps in patient and product safety. These experts should devise methods to reduce or eliminate high risks, supported by evidence.
These risk-reduction methods can be implemented on a one-time, periodic, or continuous basis. It is critical to establish and implement Standard Operating Procedures (SOPs) with regular reviews.
This must be complemented by system qualifications and the validation of production processes, including cleaning, sterilization, decontamination, and environmental monitoring. Lastly, ensuring compliance and providing personnel training are essential.
To complete the assessment, it is important to identify and gather relevant documents related to risk mitigation methods. Historical data and trends should be analyzed to evaluate necessary corrective actions and improvements that may or may not be required.
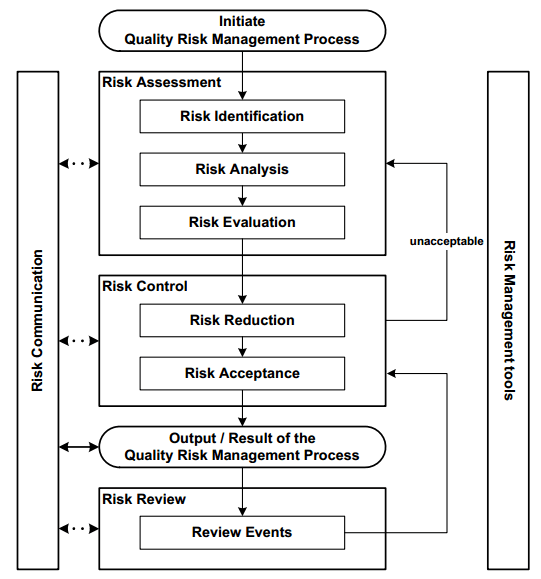
Figure 2. Overview of quality risk management (QRM) Process. Image Credit: Particle Measuring Systems
Acceptance: Compilation of CCS documentation
Compiling CCS documentation into a single document is a necessary yet challenging task. Failure to organize pertinent documents can create several issues. To start, it can hinder the identification and comprehension of contamination risks and existing risk-mitigation methods in place.
Inadequate documentation can make it challenging to determine necessary adjustments for remediation. Manufacturers may also struggle to monitor and analyze historical data, impeding the recognition of patterns and trends.
The absence of centralized documentation may result in non-compliance with industry standards and regulations or hinder the demonstration of the company’s commitment to safeguarding its products, personnel, and the environment.
Comprehensive documentation should encompass detailed information on risk assessments, protocols, and standards for preventing and mitigating contamination risks. This should include data on staff training, qualifications, equipment design, maintenance, and facility cleaning and sanitation procedures.
The documentation should also outline any testing and monitoring conducted to detect potential contamination sources.
Remediation: CCS evaluation
The 2022 Annex 1 document underscores the significance of adopting a comprehensive approach when assessing contamination prevention and control.
2.6 The CCS should consider all aspects of contamination control with ongoing and periodic review resulting in updates within the pharmaceutical quality system as appropriate. Changes to the systems in place should be assessed for any impact on the CCS before and after implementation.
Manufacturers conduct CCS evaluations after reviewing control-collected data. This analysis assesses whether the program effectively prevents contamination and whether the residual risk falls within acceptable ranges set by regulatory bodies like the FDA or EU GMP.
If this is not the case, necessary modifications and upgrades should be implemented as warranted.
The frequency and occurrence of CCS remediation depend on factors such as process and equipment complexity, facility risk levels, contamination history, and relevant legal and regulatory requirements.
Facilities introducing new equipment or a Quality Management System (QMS) may have specific review intervals. Factors like the environment, high staff turnover, deviations, external audits, and inspections also influence the review frequency.
Technical controls
Technical controls support risk communication and management tools. When adequately implemented and maintained, these technical controls enable equipment, systems, and procedures to collaborate in thwarting, identifying, and reducing the dissemination of pollutants.
Some instances of technical controls within sterile manufacturing for a CCS encompass:
- Air filtration and ventilation systems: These systems regulate airborne pollutant dissemination and uphold the appropriate flow rate in the facility.
- Cleanroom technology: Utilizing specialized equipment and construction methods to establish a regulated environment with minimal contaminants.
- Equipment and facility design: The layout and configuration of the facility are structured to minimize risks by segregating clean zones from unclean areas and employing barriers to avert cross-contamination.
- Process controls: Dedicated equipment and procedures regulate pollutant spread during manufacturing, including isolating specific processes, using specialized packaging and sealing techniques, and enforcing stringent hygiene protocols for personnel.
- Material controls: Employing specific protocols for documenting, handling, storing, and disposing of raw materials, active ingredients, and finished products to reduce contamination risks.
- Monitoring and testing: Conducting contamination testing within the facility, encompassing air and surface sampling, as well as environmental monitoring to comprehend and diminish risks.
- Maintenance and decontamination: Implementing specialized cleaning and maintenance procedures to prevent contamination and protocols for eliminating and preventing the accumulation of contaminants and residue on equipment and facilities.
- Automation: Utilizing automated systems for monitoring, recording, and controlling process parameters to reduce human errors and ensure consistent performance.
Conclusion
A CCS for pharmaceutical and biomedical manufacturing comprises a set of procedures and protocols aimed at preventing, managing, and monitoring the spread of contaminants during production.
This plays a crucial role in ensuring product safety, purity, and effectiveness. The CCS for pharmaceutical manufacturing encompasses various measures, including:
- Enforcement of stringent hygiene and sanitation protocols for facilities, equipment, and personnel
- Limiting access to specific facility areas to minimize contamination risks
- Implementation of procedures for the safe handling, storage, and disposal of supplies and hazardous byproducts
- Regular inspections and tests to detect potential contamination sources
- Formulation of contingency plans for contamination incidents
- Training of employees and contractors on contamination control procedures
- Implementation of a Quality Management System (QMS) to ensure compliance with regulations, particularly Good Manufacturing Practices (GMP)
- Regular review and updating of the CCS for continued effectiveness
- Enforcement of specific procedures for handling raw materials, active ingredients, and finished products
- Implementation of rigorous protocols for equipment and facility cleaning and maintenance
Regulatory bodies like the FDA and WHO closely oversee CCS in pharmaceutical manufacturing, with non-compliance potentially leading to substantial penalties and product recalls.
What can a PMS advisor do for you?
Engaging a Particle Measuring Systems Advisory Specialist to conduct or support a CCS audit can offer several advantages.
A comprehensive CCS audit demands expertise and objectivity. With a PMS Advisor’s service, manufacturers gain contamination control expertise, valuable insights, improvement recommendations, and an impartial CCS evaluation, often proving more effective than relying on internal personnel.
Figure 3. Quality assurance flow to support CCS. Image Credit: Particle Measuring Systems
Audits consume time, and outsourcing a CCS audit can be more cost-effective than hiring extra staff or training current staff for the task. An Advisor can swiftly complete the audit without diverting staff from their regular responsibilities.
The PMS Advisory team ensures regulatory compliance, conducts thorough risk assessments, identifies potential contamination sources, and suggests appropriate controls.
Acknowledgments
Originally authored by Ugonna Omeronyé, Global Pharma Advisory Specialist.
References
Annex 1, Manufacture of Sterile Medicinal Products -The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use - 22.8.2022 C (2022) 5938 final
ICH Guideline for Good Manufacturing Practice, Medicinal Products for Human and Veterinary Use, Step 4: GMPrelated documents, ICH guideline Q9 on Quality Risk Management (2005)
FDA Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing - Current Good Manufacturing Practice (2004)
About Particle Measuring Systems
Particle Measuring Systems has 35 years experience designing, manufacturing, and servicing microcontamination monitoring instrumentation and software used for detecting particles in air, liquid, and gas stream as well as molecular contamination monitoring.
Specific applications include cleanroom monitoring, parenteral sampling, filter and in-line testing in deionized water and process chemicals, and point-of-use monitoring of inert gases and in-situ particle monitoring. Specialty monitoring includes parts cleanliness testing with a highly automated solution.
Particle measuring systems
Whether you want to protect product or meet industry requirements, such as ISO 14644, USP 797, or GMP, Particle Measuring Systems has a large variety of particle counters and molecular monitors to meet your needs. With 35 years experience, we have the proven reliability to support your application.
Particle counters
Protect your product with our reliable particle counters. We have airborne, portable, and liquid particle counters for a wide variety of applications including DI water, chemicals, and cleanroom monitoring. Compare particle counters or learn how to monitor your cleanroom or product by reading our papers.
Molecular contamination monitors
Molecular contamination creates costly problems to high value products, production processes, and equipment surfaces. We have solutions for both Airborne Molecular contamination (AMC) and Surface Molecular contamination (SMC). With parts-per-trillion limits of detection, real-time sampling, NIST traceable calibrations, and various data analysis packages, you can monitor in confidence.
Gas detectors
If you need gas detectors for process control or continuous emissions monitoring, we can help. Get real-time, reliable results with our ammonia, hydrogen fluoride, and chlorine detectors for worker protections, CEMs, and pollution monitoring.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.