MG, an autoimmune disease, occurs when the body's immune system produces antibodies that attack vital proteins at the neuromuscular junctions, impacting the skeletal muscles responsible for breathing and various movements. It is estimated that this chronic neuromuscular disorder impacts over 700,000 individuals worldwide.1
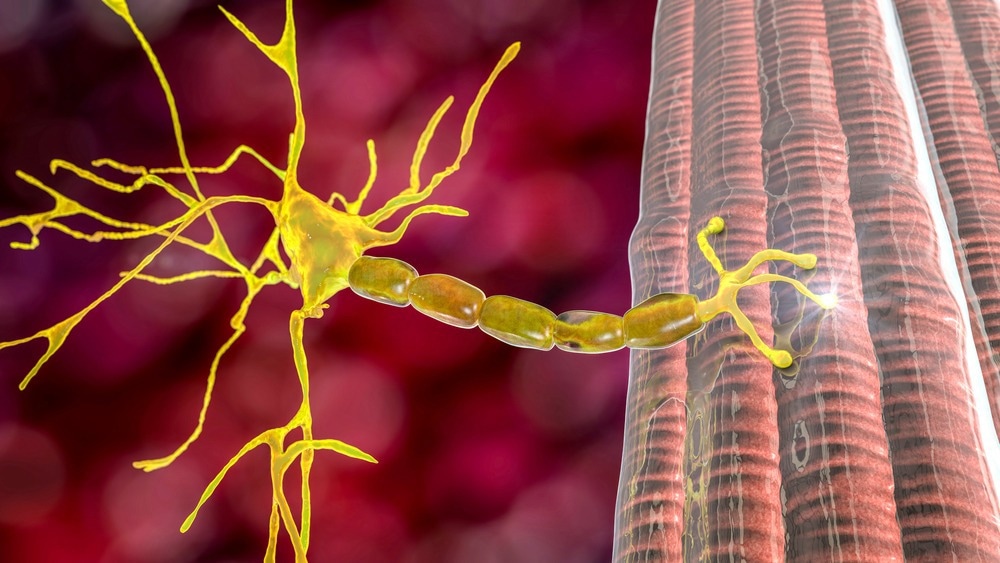
Image Credit: ShutterStock/Kateryna Kon
Early and accurate diagnosis holds immense importance as it allows for timely treatment to manage symptoms and enhance patients' quality of life, even in the absence of a cure.
Tecan, with 40 years of diagnostic expertise, offers a range of biomarkers and targeted immunoassays to identify and measure the autoantibodies associated with this condition, thereby increasing the likelihood of early detection and appropriate treatment for MG patients.
A complex condition
The primary characteristic of MG is muscle weakness, particularly affecting the muscles controlling eye and eyelid movement, facial expressions, chewing, swallowing, and speaking. This weakness tends to worsen after physical exertion and improves with rest.2
However, these symptoms often overlap with those of numerous other disorders, and the degree of muscle weakness can vary significantly from person to person, which has historically led to missed or delayed diagnoses.2
Modern disease biomarkers and targeted assays are now available to enhance the speed and reliability of diagnosis.
Image Credit: ShutterStock/luchschenF
In most MG cases, autoantibodies specifically target acetylcholine receptors, transmembrane proteins responsible for transmitting electrical signals between nerve endings and muscles to facilitate muscle contractions.3
Autoantibodies against acetylcholine receptors (ARAbs) hinder the binding of acetylcholine to its receptor, thus impeding normal muscle contractions. However, approximately 15% of patients exhibiting clinical symptoms do not show detectable ARAbs, resulting in a seronegative classification.3
About 70% of seronegative MG cases can be diagnosed by detecting autoantibodies to muscle-specific receptor tyrosine kinase (MuSK), a protein involved in linking the acetylcholine receptor to the cytoskeleton.4
Seronegative patients typically experience a more severe form of the condition, often requiring intensive therapy, with around 30% needing respiratory support. Hence, early and definitive diagnosis plays a vital role in such cases.
Leading the way in MG diagnostics
Tecan first became involved in MG diagnostics back in 2000 when the company introduced a pioneering radioreceptor assay (RRA) for detecting ARAbs.
This assay combines human muscle receptors with a radio-labeled alpha-bungarotoxin snake venom marker that specifically binds to acetylcholine receptors without interfering with ARAb binding.
The RRA assay not only boasts high sensitivity, establishing it as the gold standard for serological MG diagnosis, but it is also semi-quantitative, which holds particular significance in the context of myasthenia gravis treatment.
As immunosuppressant therapies require time to take effect, the determination of autoantibodies provides information concerning the (patho-) physiological state of the patient, providing early indications of remission or treatment failure.
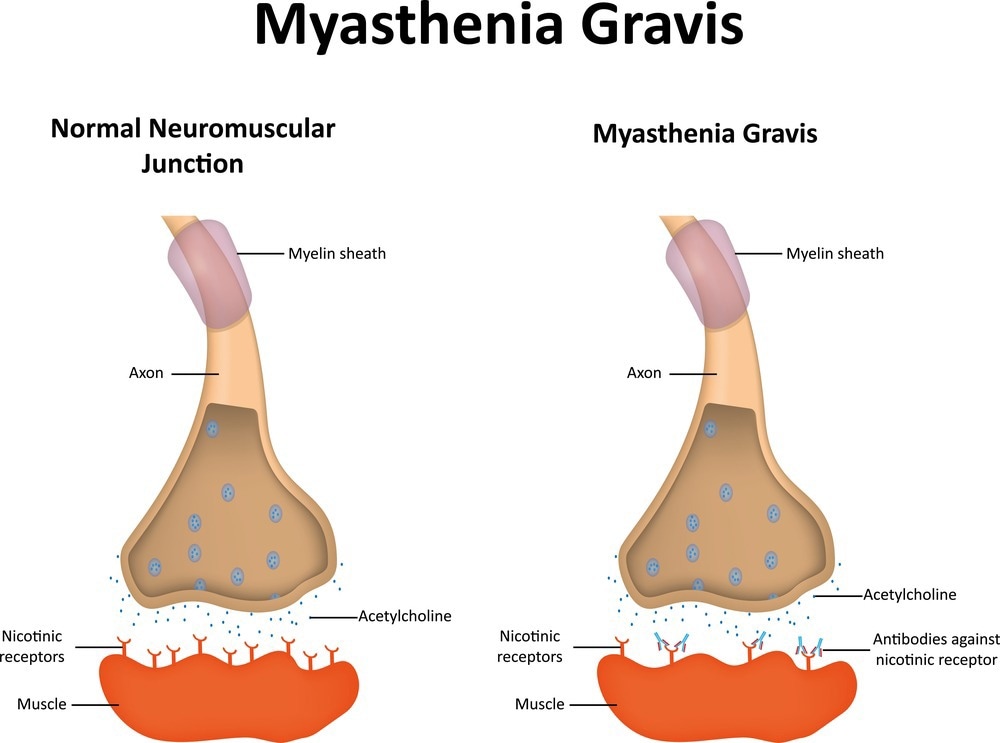
Image Credit: ShutterStock/joshya
In 2012, Tecan expanded its repertoire of MG diagnostic tools by introducing an enzyme-linked immunosorbent assay (ELISA) for detecting autoantibodies against muscle-specific receptor tyrosine kinase (MuSK).
This non-radioactive assay, the world's first commercially available assay of its kind, is designed to enhance both diagnostic certainty and allows comparison of data of individual patients by follow-up measurements during treatment to observe patients’ disease status, especially for seronegative patients.
Given the severity of this particular form of the disease, the required therapy, typically involving rituximab immunosuppression to deplete B cells, is more intensive and aims to completely eradicate anti-MuSK antibodies. Therefore, a sensitive assay for frequent analysis during treatment is crucial, and Tecan's anti-MuSK ELISA provides:
- Data integrity, with excellent clinical sensitivity (95.8%) and specificity (100 %)
- Qualitative (cut-off) and quantitative (standard curve) evaluation of results
- A holistic diagnostic solution that can be processed on open ELISA systems**
At the forefront of innovation
While a cure for MG remains elusive, the diagnosis and treatment of this condition have experienced significant advancements in the past four decades. Tecan has played a crucial role in driving these advancements by developing a comprehensive solution to diagnose both seropositive and seronegative MG patients.
Maintaining its commitment to innovation, Tecan prioritizes the development of novel solutions, ensuring its position at the forefront of modern diagnostics. Tecan has a new MG immunoassay in development, which will expand its current portfolio of diagnostic offerings.
Disclaimers
* Product availability and regulatory status may vary across regions outside the EU, depending on local country-specific registration. The assays are IVDR certified. Consult with your Tecan associate for further information.
** The combined use of the assay, process script, and instrument must be validated individually on-site by each laboratory.
References and further reading
- Sanders, D.B. et al. International consensus guidance for management of Myasthenia Gravis. Neurology, 2016, 87(4), 419-425. doi: 10.1212/wnl.0000000000002790.
- Myasthenia Gravis. National Institute of Neurological Disorders and Stroke. Available at: https://www.ninds.nih.gov/health-information/disorders/myasthenia-gravis (Accessed: 19 May 2023).
- Lazaridis, K. and Tzartos, S.J. Myasthenia gravis: Autoantibody specificities and their role in MG Management. Frontiers in Neurology, 2020, 11. doi: 10.3389/fneur.2020.596981.
- Ali, H. et al. Muscle-specific tyrosine kinase-associated myasthenia gravis: A neuromuscular surprise. Case Reports in Neurological Medicine, 2021, 1-3. doi: 10.1155/2021/1326442.
About IBL International GmbH, Part of Tecan Group
IBL International has nearly 30 years experience in development, production and sales of immunodiagnostic products, and is an expert in these fields. Our product portfolio contains enzyme-, radio- and luminescence immunoassays that covers over 1000 products in a broad range of in-vitro diagnostics for research and routine. These products are designed for manual use, but can be easily adapted for open automated systems. We also have a large selection of “niche products” for special applications to complete our product line.
IBL International also focuses on special test systems outside of the standard routine diagnostic tests. For example, we carry immunoassays for the determination of Neurotransmitters or other biogenic amines, steroid hormones in saliva, HMGB1, Neopterin and assays for Alzheimer’s research. We are continuously refining our immunoassays to assure high quality and reliable products. IBL International has a widespread distribution network in over 90 countries. We support our national and international customers with our professional team of product managers and customer service professionals.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.