Mass spectrometry (MS) is becoming increasingly prevalent in laboratories as a method of measuring small molecules, such as steroids, as well as other analytes, like biogenic amines.
Distinguishing between different small molecules when they have similar structures but distinct actions in the human body can pose a significant challenge for laboratories. For example, the steroid pathway starts with cholesterol, which is converted by specific enzymes into pregnenolone and then progesterone (Figure 1).
These two steroids differ in their biological impact but can be quite challenging to measure with immunoassays as they have structures that are so similar. This is due to the fact that immunoassays can lack specificity to distinguish subtle molecular differences between some surface antigens, despite otherwise being highly accurate and widely used for many analytes across clinical research and diagnostics.
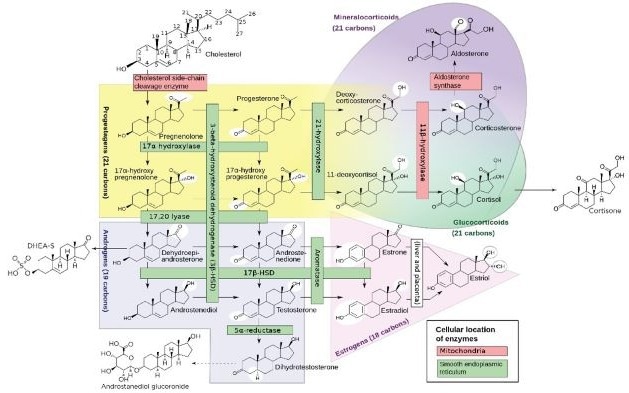
Figure 1. The steroid pathway. Reworked from the “Diagram of the pathways of human steroidogenesis”, WikiJournal of Medicine 1 (1). DOI:10.15347/wjm/2014.005. ISSN 20018762.
Overcoming the identification challenge
Clinical laboratories are looking to use MS to improve the specificity of hormone diagnostics, with liquid chromatography-mass spectrometry (LC-MS) currently recognized as the reference method for endocrinology.
MS breaks down the molecules of a sample and analyzes them by mass and charge, offering deeper analysis and clear identification of the presence of different steroid hormones.
This state-of-the-art technology makes it possible to determine and quantify a range of steroids in one assay and so provides all the necessary information to improve the quality of diagnostics.
Simultaneous measurement of all the different analytes involved in a particular steroid cascade is valuable as it allows them to, for instance, pinpoint and treat issues with a specific metabolic enzyme.
For example, an elevated 17-OH progesterone value in combination with a very low cortisol concentration suggests that the patient’s 21-hydroxylase enzyme is not acting as expected. While, theoretically, the sample could be screened by immunoassay, extra work would be needed in order to fully comprehend the patient’s condition.
18 in one
Tecan has experience in LC-MS workflows gathered from across its life sciences and diagnostics businesses and is using this expertise to offer the best and latest technologies in endocrinology to laboratories around the world. Tecan has recognized the increasing demand for MS approaches in hormone diagnostics and has responded by developing the Steroid Panel LC-MS kit*.
This CE-IVDD assay facilitates the easy measurement of 18 analytes across clinically relevant ranges – which vary for different steroids – from a single sample. Sample preparation for this assay can also be automated using the Resolvex® A200 Positive Pressure Workstation, providing a complete solution that eases the sample preparation bottleneck to save time and resources.
Your method, Tecan standards, trusted results
Some laboratories prefer to opt for their own tests over commercially available assays. Standards and control materials are still required here, and producing them creates an additional burden and takes up time and resources. The Tec-Trace* product family consists of a range of calibrators and control reagents for the calibration of LC-MS assays.
Tecan’s Steroid Panel Tec-Trace** allows reliable LC-MS quantification of the same 18 analytes as the Steroid Panel LC-MS Kit*. This solution is cost-effective, efficient, and traceable, and it can provide facilities using LC-MS tests with consistent and standardized calibration across various laboratories and LC-MS systems, supporting reproducible results and increased productivity.
Calibrators with high- and low-level controls provide independent validation of each analytical run. The Tec-Trace* range of products now includes calibrator sets for cortisol and cortisone, as well as metanephrines, methylmalonic acid, and certain vitamins.
Each calibrator set is prepared in a dedicated matrix to overcome matrix effects and supplied lyophilized for maximum lifetime, robustness, and stability.
Everything you need from a single provider
Whatever the need, be it a set of calibrators or controls, a complete test kit, use of manual sample preparation or an automated solution, Tecan is ready to support you in finding the best solution for your lab.
To find out more or to see Tecan’s full LC-MS reagents portfolio, visit https://ibl-international.com/en/lc-ms-solutions
* Availability and regulatory status may vary across regions depending on local country-specific regulation. Please consult your local representative. In the US: For research use only. Not for use in diagnostic procedures.
**For research use only. Not for use in diagnostic procedures.
Acknowledgments
- Produced from materials originally authored by Magali Wolff, Head of Global Reagent Marketing and Support at Tecan
About IBL International GmbH, Part of Tecan Group
IBL International has nearly 30 years experience in development, production and sales of immunodiagnostic products, and is an expert in these fields. Our product portfolio contains enzyme-, radio- and luminescence immunoassays that covers over 1000 products in a broad range of in-vitro diagnostics for research and routine. These products are designed for manual use, but can be easily adapted for open automated systems. We also have a large selection of “niche products” for special applications to complete our product line.
IBL International also focuses on special test systems outside of the standard routine diagnostic tests. For example, we carry immunoassays for the determination of Neurotransmitters or other biogenic amines, steroid hormones in saliva, HMGB1, Neopterin and assays for Alzheimer’s research. We are continuously refining our immunoassays to assure high quality and reliable products. IBL International has a widespread distribution network in over 90 countries. We support our national and international customers with our professional team of product managers and customer service professionals.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.