The measurement of soluble interleukin-2 receptor (sIL-2R) levels in serum or plasma in adults has become a valuable tool for clinicians to assess immune function in vivo as part of the investigation, management, or prognosis of a wide range of diseases.
Image Credit: ShutterStock/jijomathaidesigners
However, the measurement of sIL-2R is simple compared to the complex story behind this biomarker that has become so central to the study of immune-mediated disease.
Why measure soluble interleukin-2 receptor levels?
Interleukin-2 (IL-2) plays a crucial role as a signaling molecule in the human immune system. It belongs to a group of small, secreted proteins called cytokines, which have specific effects on cell interactions and communication.
IL-2 regulates the activities of white blood cells responsible for immunity, contributing to the body's natural response to infection and its ability to differentiate between foreign ("non-self") and "self" entities.1
IL-2 exerts its effects by binding to IL-2 receptors expressed by lymphocytes, with activated CD4+ T cells and CD8+ T cells being the primary sources of IL-2.1
Upon T-cell activation, the soluble form of the interleukin-2 receptor (sIL-2R) is secreted or "shed," resulting in elevated concentrations of sIL-2R in patients with various conditions associated with an ongoing immune response.1
Immune-mediated diseases linked to increased sIL-2R levels include but are not limited to, sarcoidosis, multiple sclerosis, biliary cirrhosis, chronic immune activation in common variable immunodeficiency (CVID), and hemophagocytic lymphohistiocytosis (HLH).2-6
The convenient implication of this association is that sIL-2R could potentially serve as a generic biomarker to monitor and predict disease activity and treatment response specific to these diseases and many others.7,8
However, to ensure the clinical relevance of measurements, it is necessary to delve deeper into the functioning of the IL-2/sIL-2R system.
IL-2 and sIL-2R in immune activation: Unravelling the twisted plot
The binding of IL-2 to the soluble IL-2 receptor (sIL-2R) can have varying effects on the immune response, depending on the specific target cell involved in either immunity or self-tolerance.9,10
The history of this soluble interleukin-2 receptor, also known as sIL-2R, sCD25, sTAC, sIL2R, or IL-2RA, dates back to 1985 when it was initially described as actively released by activated peripheral blood T-cells through proteolytic cleavage of the IL-2R present on the cell surface.11
In 1990, Rubin and colleagues were the first to demonstrate that upon in vitro activation, T lymphocytes not only increased cellular IL-2R expression but also released soluble IL-2R(α). They found that similar to cellular IL-2R expression, the release of soluble IL-2R necessitated de novo protein synthesis rather than cellular proliferation.12
Despite the long-established association between immune activation and elevated sIL-2R release under pathological conditions, the precise biological actions of sIL-2R are still not fully understood.
Several mechanisms of action have been proposed, ranging from immune-inhibitory to immuno-stimulatory effects. Figure 1 provides a glimpse into the paradoxical and intricate nature of the IL-2/sIL-2R system.10
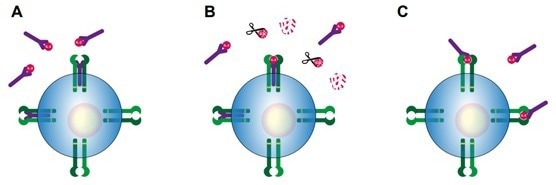
Figure 1. Potential mechanisms of action of sIL-2R during immune activation, as proposed by Dik & Heron (2020).10 A) sIL-2R binds IL-2, thereby prohibiting IL-2 from binding to either to the low affinity dimeric IL-2 receptor or high affinity trimeric IL-2 receptor. B) sIL-2R binds IL-2, thereby protecting IL-2 from enzymatic degradation and prolonging IL-2 half-life. C) sIL-2R binds IL-2, thereby increasing the affinity of IL-2 for the low affinity dimeric IL-2 receptor. IL-2Rα: purple, IL-2Rβ: light green, IL-2Rγ: dark green, sIL-2R: purple.
Image Credit: Tecan
Soluble IL-2R binds to IL-2 with high efficiency, and in vitro experiments have suggested that sIL-2R might limit the activation and proliferation of T lymphocytes by sequestering available IL-2.13
However, conflicting data has been reported.14 Alternatively, when complexed with IL-2, sIL-2R may prolong the half-life of IL-2, potentially enhancing its immune-stimulatory properties, even by activating low-affinity dimeric IL-2R.15
sIL-2R can present IL-2 to CD4+ T lymphocytes, leading to their differentiation into regulatory T cells (Tregs) instead of T-helper (Th)1 or Th17 lymphocytes, which can subsequently suppress immune activity.16
On the other hand, there are reports supporting the notion that sIL-2R may contribute to (auto)immune processes by promoting enhanced Th17 generation, which involves sequestering the IL-2 that normally inhibits early Th17 differentiation.17
Despite the evident complexity and lack of consensus regarding the precise mechanism(s) of action of different forms of sIL-2R, as well as their occurrence and final biological effects in vivo, the currently available data at least establishes a role for sIL-2R in the regulation of IL-2-dependent cell function.
What makes sIL-2R an appealing biomarker?
Earlier, it was observed that elevated blood levels of sIL-2R are indicative of an ongoing immune response, which could potentially serve as a monitoring tool for a wide range of immune-mediated diseases. This initial concern about the generic nature of the response should not overshadow its significance.9
In general, conditions characterized by excessive production of lymphocytes, known as lymphoproliferative disorders, exhibit higher sIL-2R levels compared to healthy individuals. Similarly, granulomatous diseases like sarcoidosis, where T-cell activation is a typical characteristic, also demonstrate elevated sIL-2R levels.
The consistent stability of sIL-2R levels throughout adulthood, along with minimal gender-related variations, further enhances its appeal as a biomarker.12,18
However, it is important to consider individual variations in factors such as age, gender, lifestyle, and overall health when determining reference values and ranges.19
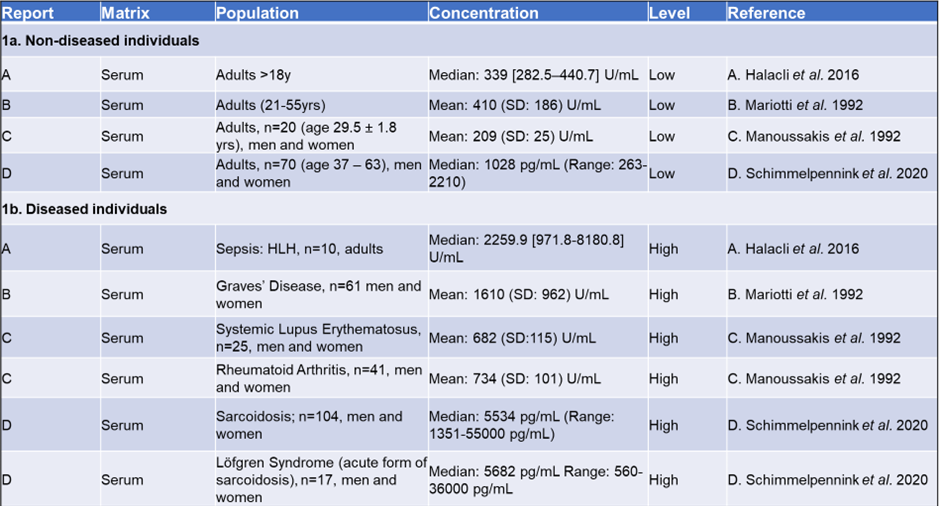
Table 1 provides an overview of sIL-2R levels in both healthy and diseased individuals.
Since there is no universally recognized standard set by the World Health Organization (WHO), the measurements presented in this table are dependent on the specific assay used. Nevertheless, it is recommended to calibrate assay kits against the international reference standard NIBSC 97/600.
When comparing relative sIL-2R levels, it can be reasonably concluded that diseased individuals exhibit higher sIL-2R levels compared to healthy populations, as shown in Table 1.
Table 1. Comparing levels of sIL-2 receptor in healthy and diseased individuals. 1a: Reference ranges for sIL-2R in an apparently healthy population measured in serum and plasma in adults. 1b: sIL-2R values for affected population measured in serum and plasma. (letters A-D indicate publication) Source: Tecan
How do we measure soluble interleukin-2 receptor?
Soluble IL-2 receptor levels are typically assessed through immunoassays, namely enzyme-linked immunosorbent assay (ELISA) or chemiluminescent immunoassay (CLIA).
Both assay types exhibit a strong correlation in their results, although ELISA yields absolute values (pg/mL) approximately 7-8 times higher than CLIA (U/mL). To ensure accurate measurements, commercially available ELISAs should be calibrated against the international reference standard NIBSC 97/600.
ELISA assays for sIL-2R are typically designed for batch analysis, with a measuring range of up to 10,000-20,000 pg/mL based on a standard serum dilution of approximately 1:5.
By employing automated liquid-handling systems, multiple dilutions can be analyzed simultaneously, enabling the quantification of higher sIL-2R levels.
In clinical practice, different criteria must be employed to determine the upper limit of normal (cut-off) for sIL-2R levels due to the wide range of values (refer to Table 1).
These will vary depending on the specific disease being diagnosed or monitored and the disease stage at which sIL-2R levels are measured.
While numerous Research Use Only (RUO) ELISA kits are available for measuring sIL-2R levels, it is crucial to ensure compliance with applicable regulations when using a kit for diagnostic purposes. In the European Union, this means that kits used for diagnosis must conform to IVDR (Regulation (EU) 2017/746 for in vitro diagnostic medical devices).
The principal characteristics of sIL-2 kits might include, but are not limited to:
- All kit reagents should be ready-to-use – no dilution necessary. Include ready-to-use standard-curve reagents – no stock standard to dilute.
- Kit ready-calibrated against NIBSC 97/600 Standard Preparation
- Internal kit controls provided.
- Easily automatable.
Sarcoidosis and sIL-2R: A deep dive
Sarcoidosis, also referred to as Besnier-Boeck-Schaumann disease, is a rare ailment that triggers the formation of granulomas, small swollen tissue patches, in various organs of the body.24
It commonly affects the lungs and lymph nodes and can also impact the skin. The symptoms of sarcoidosis vary depending on the organs involved but typically include tender skin bumps, breathlessness, and a persistent cough.
Predicting the specific impact of sarcoidosis on an individual is impossible since the condition can affect any organ, resulting in a wide range of symptoms.
In some cases, sarcoidosis may be acute or chronic, and individuals may not exhibit any symptoms, leading to diagnosis through incidental findings on an X-Ray examination.
Image Credit: ShutterStock/ALIOUI MA
Numerous studies have reported elevated levels of sIL-2R in sarcoidosis patients, establishing it as a recognized biomarker for the disease.2,25 Some studies suggest that measuring sIL-2R can serve as an indicator of treatment success.26
The sensitivity of serum sIL-2R as a diagnostic biomarker for sarcoidosis is around 79%, and in patients with uveitis, the sensitivity of elevated sIL-2R levels in identifying underlying sarcoidosis is approximately 81%–98%, with an AUC of 0.76 (fair) and 0.96 (excellent).27
Notably, patients with extrapulmonary involvement tend to have relatively high levels of serum sIL-2R, indicating its potential as a biomarker for staging and/or assessing disease severity.
Advanced radiographic stages and progressive disease are associated with higher sIL-2R levels. Compared to soluble angiotensin-converting enzyme (sACE), serum sIL-2R tests demonstrate superior ability in determining pulmonary severity.
Additionally, unlike sACE, the interpretation of serum sIL-2R levels is not affected by drug or immunosuppressant use.27
It should be noted that increased serum sIL-2R values are not always specific to sarcoidosis, as elevated values can be observed in other conditions such as hematologic malignancies, other granulomatous diseases, various autoimmune disorders, and post-transplantation scenarios.
Renal insufficiency can also impact sIL-2 levels, leading to potential misinterpretation of test results.27
Nevertheless, when considering the overall clinical picture of an individual patient, measuring serum sIL-2R remains a valuable prognostic marker.
High levels of serum sIL-2R can predict the need for therapy in sarcoidosis patients, and elevated sIL-2R at the start of therapy has shown promise as a predictor of relapse following treatment with infliximab.27
Changes in serum sIL-2R concentration correlate well with clinical changes, pulmonary function parameters, and radiological abnormalities, making serial measurements of serum sIL-2R beneficial for assessing disease activity during sarcoidosis follow-up.27
In conclusion, when it comes to establishing a reliable tool for diagnosing, monitoring, and treating sarcoidosis, the measurement of soluble interleukin-2 receptor (sIL-2R) is a permanent fixture.
The future of sIL-2R measurement
It has been observed that the measurement of soluble interleukin-2 receptor (sIL-2R) can serve as an immensely valuable tool for clinicians in assessing immune function in vivo, spanning a wide range of diseases and establishing its crucial role in investigating and managing sarcoidosis.
Image Credit: ShutterStock/Magic mine
The widespread adoption of sIL-2R measurement as a standard practice in the clinical setting will, of course, rely on the development of assays compliant with IVDR.
Nevertheless, a significant portion of the groundwork necessary for their introduction into the clinic has been accomplished, and sIL-2R ELISA kits are already accessible for research purposes.
As more efforts are focused on uncovering the potential applications of this ubiquitous and intricate biomarker, it can aid in diagnosing and monitoring autoimmune diseases.
References and further reading
- Liao, W., Lin, J. X., & Leonard, W. J. (2011). IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Current opinion in immunology, 23(5), 598–604. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/21889323/ DOI: https://doi.org/10.1016/j.coi.2011.08.003
- Thi Hong Nguyen, C., Kambe, N., Kishimoto, I., Ueda-Hayakawa, I., & Okamoto, H. (2017). Serum soluble interleukin-2 receptor level is more sensitive than angiotensin-converting enzyme or lysozyme for diagnosis of sarcoidosis and may be a marker of multiple organ involvement. The Journal of dermatology, 44(7), 789–797. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/28295528/ DOI: https://doi.org/10.1111/1346-8138.13792
- Peerlings, D., Mimpen, M., & Damoiseaux, J. (2021). The IL-2–IL-2 receptor pathway: Key to understanding multiple sclerosis. Journal of Translational Autoimmunity, 100123. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/35005590/ DOI: https://doi.org/10.1016/j.jtauto.2021.100123
- Barak, V., Selmi, C., Schlesinger, M., Blank, M., Agmon-Levin, N., Kalickman, I., Gershwin, M. E., & Shoenfeld, Y. (2009). Serum inflammatory cytokines, complement components, and soluble interleukin 2 receptor in primary biliary cirrhosis. Journal of autoimmunity, 33(3-4), 178–182. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/19846277/ DOI: https://doi.org/10.1016/j.jaut.2009.09.010
- Litzman, J., Nechvatalova, J., Xu, J., Ticha, O., Vlkova, M., & Hel, Z. (2012). Chronic immune activation in common variable immunodeficiency (CVID) is associated with elevated serum levels of soluble CD14 and CD25 but not endotoxaemia. Clinical and experimental immunology, 170(3), 321–332. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/23121673/ DOI: https://doi.org/10.1111/j.1365-2249.2012.04655.x
- Lin, M., Park, S., Hayden, A., Giustini, D., Trinkaus, M., Pudek, M., Mattman, A., Schneider, M., & Chen, L. (2017). Clinical utility of soluble interleukin-2 receptor in hemophagocytic syndromes: a systematic scoping review. Annals of hematology, 96(8), 1241–1251. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/28497365/ DOI: https://doi.org/10.1007/s00277-017-2993-y
- Durda, P., Sabourin, J., Lange, E. M., Nalls, M. A., Mychaleckyj, J. C., Jenny, N. S., Li, J., Walston, J., Harris, T. B., Psaty, B. M., Valdar, W., Liu, Y., Cushman, M., Reiner, A. P., Tracy, R. P., & Lange, L. A. (2015). Plasma Levels of Soluble Interleukin-2 Receptor α: Associations With Clinical Cardiovascular Events and Genome-Wide Association Scan. Arteriosclerosis, thrombosis, and vascular biology, 35(10), 2246–2253. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/26293465/ DOI: https://doi.org/10.1161/ATVBAHA.115.305289
- Karim, A. F., Eurelings, L., Bansie, R. D., van Hagen, P. M., van Laar, J., & Dik, W. A. (2018). Soluble Interleukin-2 Receptor: A Potential Marker for Monitoring Disease Activity in IgG4-Related Disease. Mediators of inflammation, 2018, 6103064. PubMed ID: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5854105/ DOI: https://doi.org/10.1155/2018/6103064
- Damoiseaux J. (2020). The IL-2 - IL-2 receptor pathway in health and disease: The role of the soluble IL-2 receptor. Clinical immunology (Orlando, Fla.), 218, 108515. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/32619646/ DOI: https://doi.org/10.1016/j.clim.2020.108515
- Dik, W. A., & Heron, M. (2020). Clinical significance of soluble interleukin-2 receptor measurement in immune-mediated diseases. The Netherlands journal of medicine, 78(5), 220–231. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/33093245/
- Rubin, L. A., Kurman, C. C., Fritz, M. E., Biddison, W. E., Boutin, B., Yarchoan, R., & Nelson, D. L. (1985). Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. Journal of immunology (Baltimore, Md. : 1950), 135(5), 3172–3177. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/3930598/
- Rubin, L. A., & Nelson, D. L. (1990). The soluble interleukin-2 receptor: biology, function, and clinical application. Annals of internal medicine, 113(8), 619–627. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/2205142/ DOI: https://doi.org/10.7326/0003-4819-113-8-619
- Rubin, L. A., Jay, G., & Nelson, D. L. (1986). The released interleukin 2 receptor binds interleukin 2 efficiently. Journal of immunology (Baltimore, Md. : 1950), 137(12), 3841–3844. PubMed: https://pubmed.ncbi.nlm.nih.gov/3023487/
- Pedersen, A. E., & Lauritsen, J. P. (2009). CD25 shedding by human natural occurring CD4+CD25+ regulatory T cells does not inhibit the action of IL-2. Scandinavian journal of immunology, 70(1), 40–43. PubMed: https://pubmed.ncbi.nlm.nih.gov/19522766/ DOI: https://doi.org/10.1111/j.1365-3083.2009.02268.x
- Vanmaris, R. M. M., & Rijkers, G. T. (2017). Biological role of the soluble interleukin-2 receptor in sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG, 34(2), 122–129. PubMed ID: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7170137/ DOI: https://doi.org/10.36141/svdld.v34i2.5369
- Yang, Z. Z., Grote, D. M., Ziesmer, S. C., Manske, M. K., Witzig, T. E., Novak, A. J., & Ansell, S. M. (2011). Soluble IL-2Rα facilitates IL-2-mediated immune responses and predicts reduced survival in follicular B-cell non-Hodgkin lymphoma. Blood, 118(10), 2809–2820. PubMed ID: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3172797/ DOI: https://doi.org/10.1182/blood-2011-03-340885
- Maier, L. M., Anderson, D. E., Severson, C. A., Baecher-Allan, C., Healy, B., Liu, D. V., Wittrup, K. D., De Jager, P. L., & Hafler, D. A. (2009). Soluble IL-2RA levels in multiple sclerosis subjects and the effect of soluble IL-2RA on immune responses. Journal of immunology (Baltimore, Md. : 1950), 182(3), 1541–1547. PubMed ID: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3992946/ DOI: https://doi.org/10.4049/jimmunol.182.3.1541
- Taniguchi, T., & Minami, Y. (1993). The IL-2/IL-2 receptor system: a current overview. Cell, 73(1), 5–8. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/8462103/ DOI: https://doi.org/10.1016/0092-8674(93)90152-g
- Vanessa Alende-Castro, Manuela Alonso-Sampedro, Carmen Fernández-Merino, Bernardo Sopeña, Carmen Vidal, Francisco Gude & Arturo Gonzalez-Quintela (2023). Factors influencing serum concentrations of soluble interleukin-2 receptor: a general adult population study, All Life, 16:1, 2169958 DOI: https://doi.org/10.1080/26895293.2023.2169958
- Halacli, B., Unver, N., Halacli, S. O., Canpinar, H., Ersoy, E. O., Ocal, S., Guc, D., Buyukasik, Y., & Topeli, A. (2016). Investigation of hemophagocytic lymphohistiocytosis in severe sepsis patients. Journal of critical care, 35, 185–190. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/27481757/ DOI: https://doi.org/10.1016/j.jcrc.2016.04.034
- Mariotti, S., Caturegli, P., Barbesino, G., Marinò, M., Del Prete, G. F., Chiovato, L., Tonacchera, M., De Carli, M., & Pinchera, A. (1992). Thyroid function and thyroid autoimmunity independently modulate serum concentration of soluble interleukin 2 (IL-2) receptor (sIL-2R) in thyroid diseases. Clinical endocrinology, 37(5), 415–422. PubMed ID : https://pubmed.ncbi.nlm.nih.gov/1486691/ DOI: https://doi.org/10.1111/j.1365-2265.1992.tb02352.x
- Manoussakis, M. N., Germanidis, G. S., Drosos, A. A., & Moutsopoulos, H. M. (1992). Impaired urinary excretion of soluble IL-2 receptors in patients with systemic lupus erythematosus and rheumatoid arthritis. Lupus, 1(2), 105–109. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/1301961/ DOI: https://doi.org/10.1177/096120339200100208
- Schimmelpennink, M. C., Quanjel, M., Vorselaars, A., Wiertz, I., Veltkamp, M., Van Moorsel, C., & Grutters, J. C. (2020). Value of serum soluble interleukin-2 receptor as a diagnostic and predictive biomarker in sarcoidosis. Expert review of respiratory medicine, 14(7), 749–756. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/32248706/ DOI: https://doi.org/10.1080/17476348.2020.1751614
- https://www.nhs.uk/conditions/sarcoidosis/ Accessed 13 April 2023
- 25. Eurelings, L. E. M., Miedema, J. R., Dalm, V. A. S. H., van Daele, P. L. A., van Hagen, P. M., van Laar, J. A. M., & Dik, W. A. (2019). Sensitivity and specificity of serum soluble interleukin-2 receptor for diagnosing sarcoidosis in a population of patients suspected of sarcoidosis. PloS one, 14(10), e0223897. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/31622413/ DOI: https://doi.org/10.1371/journal.pone.0223897
- Vorselaars, A. D., van Moorsel, C. H., Zanen, P., Ruven, H. J., Claessen, A. M., van Velzen-Blad, H., & Grutters, J. C. (2015). ACE and sIL-2R correlate with lung function improvement in sarcoidosis during methotrexate therapy. Respiratory medicine, 109(2), 279–285. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/25496652/ DOI: https://doi.org/10.1016/j.rmed.2014.11.009
- Milou C. Schimmelpennink MD, Adriane D.M. Vorselaars MD, PhD, Jan C. Grutters MD. Sarcoidosis: A Clinician's Guide (2019), Chapter 19 - Biomarkers in Sarcoidosis. Pages 219-238. PubMed ID: https://pubmed.ncbi.nlm.nih.gov/25496652/ DOI: https://doi.org/10.1016/B978-0-323-54429-0.00019-7
About IBL International GmbH, Part of Tecan Group
IBL International has nearly 30 years experience in development, production and sales of immunodiagnostic products, and is an expert in these fields. Our product portfolio contains enzyme-, radio- and luminescence immunoassays that covers over 1000 products in a broad range of in-vitro diagnostics for research and routine. These products are designed for manual use, but can be easily adapted for open automated systems. We also have a large selection of “niche products” for special applications to complete our product line.
IBL International also focuses on special test systems outside of the standard routine diagnostic tests. For example, we carry immunoassays for the determination of Neurotransmitters or other biogenic amines, steroid hormones in saliva, HMGB1, Neopterin and assays for Alzheimer’s research. We are continuously refining our immunoassays to assure high quality and reliable products. IBL International has a widespread distribution network in over 90 countries. We support our national and international customers with our professional team of product managers and customer service professionals.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.