Sponsored Content by ScitegrityReviewed by Olivia FrostJun 6 2024
Legislators frequently propose and implement stringent controls on new chemicals, and it is imperative that chemical producers and users are able to proactively respond to these new controls in a timely manner.

Image Credit: Shutterstock.com/Vector_Artist
Industry is often required to quickly confirm whether its chemicals and processes are impacted and to adapt these as quickly as possible. New proposals and controls may also impact chemicals in a way that is entirely unrelated to the manner initially intended.
This article explores several scenarios where both proposed and implemented legislation to control narcotics has led to controls being necessitated on unrelated but otherwise useful chemicals, including clinical trial candidates and potentially licensed medicines.
In January 2024, the UK Government and Advisory Council on the Misuse of Drugs (ACMD) approached Scitegrity and asked it to assist in a technical evaluation of a new proposed generic statement designed to control Acyl Piperazines.
The ACMD sought to explore and prevent a repeat of previous issues encountered with the 2016 amendment for third-generation cannabinoids. In this instance, a poorly worded generic statement had resulted in a medically useful chemical space unrelated to cannabinoids becoming controlled, causing unanticipated problems for legitimate research, clinical trials, trade, and end-use of certain chemicals.
Working to strict deadlines, Scitegrity assessed the proposed Acyl Piperazines statement against a database of 10 million commercially available chemicals to evaluate the potential for ‘collateral damage’ like that seen previously with third-generation cannabinoids.
Fortunetly the impact was assessed as likely to be low, with very few chemicals flagged and those there were clearly related to Acyl Piperazine Bucinnazine and likely to be of abuse concern.
The specific wording of the assessment was as follows:
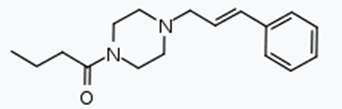
Image Credit: Scitegrity
Bucinnazine (1-butyryl-4-cinnamyl piperazine) and any compound structurally derived from bucinnazine by modification in any of the following ways,
- By substitution to any extent by methyl in the piperazine or phenyl rings
- By replacement of the butyryl group by another acyl group containing three, four, or five carbon atoms
- By substitution in the phenyl ring by a nitro group
- By addition of an ethylenic bridge across the piperazine ring
While the changes were found to have little impact on this assessment, it should not be assumed that this will always be the case.
For example, an amendment to UK legislation was proposed recently to control Cumyl-PeGaClone-related chemicals (a type of cannabinoid) due to their potential for abuse.
An analysis by Scitegrity undertaken on behalf of several of our clients identified considerable unforeseen and unintended effects related to this proposed change, including controlling hundreds of thousands of research and development chemicals unrelated to Cumyl-PeGaClone.
Many of these chemicals do not exhibit cannabinoid activity, such as over-the-counter medicines like Clotrimazole (CanestenTM).
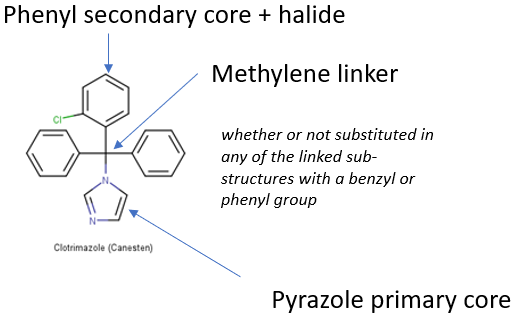
Figure 1. The proposed wording of the chemical space for Cumyl-PeGaClone unfortunately would also have controlled CanestenTM . Image Credit: Scitegrity
Approximately 20 million chemicals were assessed as part of this analysis, including a full assessment of the chemical collections of some of our clients —chemicals central to research and development and manufacturing activities.
Early feedback was provided to regulators and their advisors after the assessment, highlighting the unintended impact of the proposed changes before they began progressing through the legislative process.
Scitegrity also assessed chemical space related to nitazene-based opioids following a proposal to control nitazene related chemicals in the UK. It was noted that these proposals were unlikely to cause major issues for research in the pharmaceutical and chemicals industries.
In this instance, the chemical space in question had well-defined cores and attached chemical groups, with proposed controls unlikely to capture unrelated chemicals.
It is encouraging that regulators are beginning to be more cautious about proposed controls and are seeking to minimize unintended impacts when controlling areas of chemical space.
Scitegrity’s tools and services are ideal for this purpose, and the company encourages regulators and companies to employ its services in assessments similar to those outlined in this article.
Acknowledgments
Produced from materials originally authored by Joe Bradley from Scitegrity Limited.
About Scitegrity
Want to know if your chemical is controlled, regulated, has the potential for abuse or just need a tariff code?
Our regulatory and chemistry experts encode chemical regulations from around the world allowing you to simply answer these questions and more by drawing or looking up a chemical structure.
We make regulatory compliance a simple, robust, background process. Join with 5 of the worlds top 10 pharma, chemical suppliers, regulators, CROs, forensics labs and more who trust and rely on our solutions.
Scitegrity was founded in 2011 by ex-Pfizer, GSK and Roche chemists and data scientists with the goal of making compliance to chemical regulations are far more robust, accurate and automatic.
By automatically checking all the chemicals an organisation has at the structure level, it allows enterprise wide automatic compliance checks against hundreds of regulations globally, even for novel and proprietary chemical collection running into millions of chemicals.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.