Endocannabinoids (ECs) and steroid hormones are endogenous substances that each play a central role in human stress response regulation.
These regulators’ concentration fluctuates significantly throughout the day, making it difficult to properly correlate their relationship to the range of human stress-related conditions. Analyzing these substances is also challenging from an analytical perspective, primarily due to their chemical similarity and comparatively low levels in biological matrices.
There is, therefore, a need for sensitive analytical techniques able to accurately quantify these stress markers in biological matrices.
It is possible to detect these endogenous markers in many biological matrices, including blood, saliva, urine, and keratinized matrices such as nails and hair. Nails and hair, in particular, are becoming especially valuable in investigating the long-term and retrospective determination of endogenous stress markers because these are known to gradually accumulate in the keratinized matrix over time.
Other benefits include no storage requirements, non-invasive sample collection, long-term stability, and low risk of sample degradation over time.
This article explores a reliable, highly sensitive analytical workflow that leverages a supported-liquid extraction (SLE)- based sample preparation procedure and the SCIEX 7500 system. This robust combination enabled the detection of a panel of analytes consisting of five steroid hormones and four ECs at the sub picogram/mg level.
Results from the SCIEX 7500 system and the QTRAP 6500+ were compared. The latter is a widely recognized and highly regarded platform routinely employed in the low-level quantification of endogenous species.
Significant gains in peak area and signal-to-noise ratio were observed on the SCIEX 7500 system, showcasing its benefits when sensitively quantifying low-level stress markers in keratinized matrices.
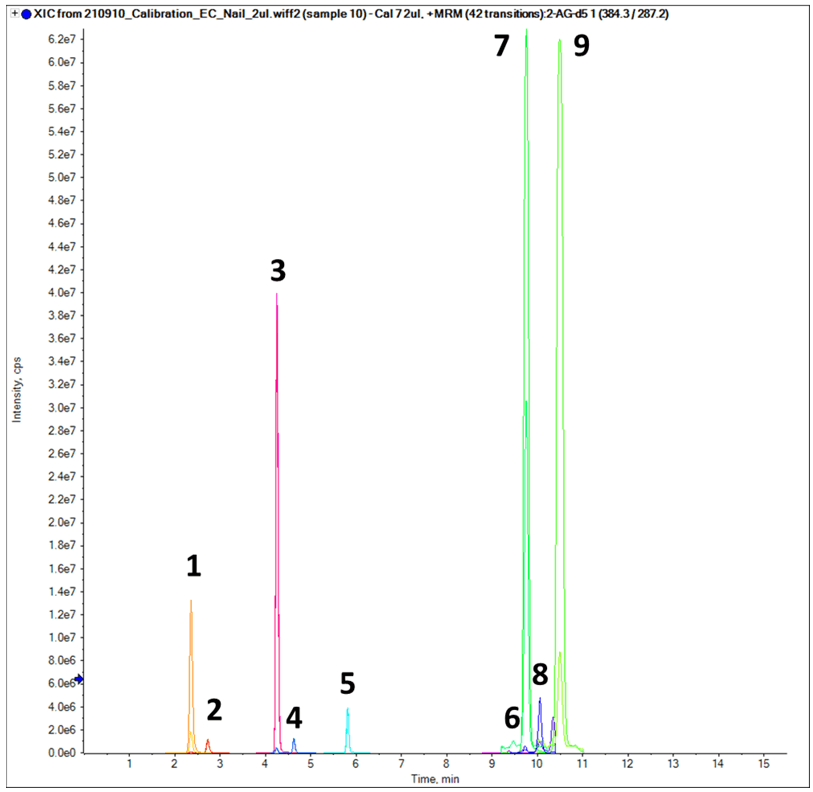
Figure 1. Chromatographic profile of the 5 steroid hormones and 4 endocannabinoids targeted in this study. Extracted ion chromatogram (XIC) resulting from the baseline separation of the 9 compounds in the panel. The numbered peaks are assigned as follows: 1. cortisone, 2. cortisol, 3. androstenedione, 4. testosterone, 5. progesterone, 6. AEA, 7. PEA, 8. 2-AG, and 9. N-OEA. The total LC runtime was 15.5 min. Image Credit: SCIEX
Advantages of the SCIEX 7500 system for high-sensitivity stress marker analysis in keratinized matrices
The sub pg/mg detection of endogenous steroid hormones and ECs in keratinized matrices is possible when using a washing procedure followed by a SLE-based sample preparation method and the use of a powerful detection method via the SCIEX OS software’s Scheduled MRM algorithm.
The SCIEX 7500 system's sensitivity gains improved the quantification of low-level stress markers in keratinized matrices.
Experimental details
Target analytes
Target analytes in this experiment included:
- Oleoylethanolamide, palmitoylethanolamide and the deuterated internal standards cortisone-D7, progesterone-D9, androstenedione, 13C3-testosterone and 13C3-progesterone purchased from Millipore Sigma (Saint Louis, USA)
- Anandamide, 2-arachidonylglycerol and the deuterated internal standard (AEA-D11) purchased from Cayman Chemicals (Ann Arbor, USA)
- 13C3-cortisol and 13C3-cortisone purchased from Isoscience (Ambler, USA)
Two solutions were prepared in acetonitrile. This comprised a standard mixture that included the nine target analytes and an internal standard mixture that included the nine deuterated internal analog standard mixtures. Both solutions were stored at -20 ºC before use.
Calibrator preparation
Calibrator preparation for this experiment was done by preparing seven levels of calibrators. This was done by spiking the deuterated internal analog standard mixture in nails and hairs across a range of concentrations as follows:
- 5 to 200 pg/mg for 2-AG D5, 0.1 and 10 pg/mg for AEA D4 and testosterone 13C3
- 1 and 500 pg/mg for androstenedione 13C3, cortisone 13C3 and progesterone 13C3
- 500 and 10000 pg/mg for N-OEA D4
- 0.5 to 50 pg/mg for cortisol 13C3
All hair and nail samples were washed for three minutes with deionized water before being washed for two minutes with acetone.
The tubes containing the hair and nail samples were vigorously shaken by hand during the washing steps. Following each washing step, the washing solutions were decanted and discarded. Finally, all washed hair and nail samples were left to dry overnight at room temperature.
Hair and nail sample preparation
The samples were cut into snippets before 20 mg of hair was weighed into an Eppendorf tube.
A total of 20 mg of nail clippings was also weighed into an Eppendorf tube before three 5 mm stainless steel milling balls were added. The nail clippings were pulverized for 10 minutes at 30 Hz.
A 1 mL quantity of methanol and 50 uL of each internal standard calibrator solution were added to each hair or nail clippings tube. These tubes were shaken briefly before being placed in a sonicated bath (35 kHz, 600 w) for 4 hours at 55 ºC to enable extraction. Next, the tubes were centrifuged at 9000 g for five minutes before the methanolic extracts were transferred to a column rack for SLE.
SLE procedure
An automated Biotage Extrahera system, (Biotage, Sweden) was used to perform the extraction. This system automatically loaded sample extracts onto Isolute SLE+ columns and these were allowed to absorb for 5 minutes.
Next, analytes were eluted twice with 2.5 mL ethyl acetate, with a 5-minute wait in between elutions. Extracts were dried using a Turbovap (Biotage, Sweden) before being resuspended in 60 µL of methanol and 140 µL of a reconstitution solution that comprised 0.2 mM ammonium formate in water:MeOH (97:3, v/v).
Liquid chromatography
HPLC separation was performed using a Phenomenex Kinetex XB-C18 (50 x 2.10 mm, 2.6 µm, 00B-4496-AN) held at 40 ºC on a Prominence UFLC system on the QTRAP 6500+ and a Nexera 40 Serie UHPLC system on the SCIEX 7500 system.
The A and B mobile phases comprised 0.2 mM ammonium formate in water:methanol (97:3, v/v) and water:methanol (3:97, v/v), respectively. The LC flow rate was 0.4 mL per minute while the total run time was 20 minutes. The injection volume was 2 µL.
Mass spectrometry
In this instance, a SCIEX 7500 system fitted with an OptiFlow Pro ion source, an electrospray ionization (ESI) analytical probe, and an E Lens probe was used. For comparison purposes, a QTRAP 6500+ system equipped with an IonDrive Turbo V ion source was also used.
Each instrument was operated in positive ESI mode and optimized to ensure maximum sensitivity.
Source parameters, as well as compound-dependent parameters for all compounds and their corresponding internal standards were also optimized on each individual system, including the Q0D dissociation on the SCIEX 7500 system.
Data acquisition and processing
Data was acquired using the SCIEX OS software on the SCIEX 7500 system and the Analyst software version 1.7 on the QTRAP 6500+ system.
All data was processed using the SCIEX OS software, with both detection and integration of the peaks from the background performed within the viewing window thanks to the MQ4 algorithm. The Analytics module of SCIEX OS software features a peak-to-peak algorithm that was used for signal-to-noise calculations.
Analytical methodology for robust, accurate quantification of low-level stress markers in keratinized matrices
A diluted, 10 ng/mL neat standard mixture containing the nine target analytes was used in the initial method development. Figure 1 features the chromatographic profile of the five steroid hormones and four endocannabinoids targeted in the study presented here.
Thanks to the combination of ideal gradient, satisfactory mobile phase composition, and appropriate column choice (Phenomenex Kinetex XB-C18), baseline separation of the nine analytes was achieved in a 20-minute total run time.
A notable challenge in detecting and accurately quantifying endogenous compounds within biological matrices is that varying levels of these analytes are already present in the matrices themselves.
A typical strategy for overcoming this analytical challenge is using surrogate analytes like heavy-labeled analogs. This approach uses a stable-isotope-labeled standard as a surrogate analyte to facilitate calibration in the genuine biological matrix.
This approach has documented uses in accurately quantifying endogenous steroids and endocannabinoids in keratinized matrices.1
Samples of real human hair and nails were spiked with the nine deuterated internal analog standards at concentrations ranging from 0.1 to 10000 pg/mg. These were then extracted using the SLE procedure before being injected into the Nexera 40 Series UHPLC system on the SCIEX 7500 system to develop a data processing method.
The calibrator solutions were injected to assess the system’s quantification performance and capacity to accurately and precisely measure low levels of drugs and their metabolites.
Figure 2 features representative extracted ion chromatograms (XICs) for two MRM transitions monitored for:
- AEA-D4 extracted from hair samples
- Progesterone 12C3 extracted from nail samples.
The XIC displays overlay both the quantifier and qualifier ions for a blank injection (left) and concentrations ranging from 0.1 to 10 pg/mg for AEA-D4 and 1 to 500 pg/mg for progesterone 12C3.
Figure 2 also shows tolerance in the ion ratio line overlay, helping to visualize confidence levels. The ion ratio difference was found to be <20% for the quantifier and qualifier ions of each target analytes across the calibration range.
It was noted that overall, the developed method was found to facilitate robust, accurate quantification of endogenous steroids and endocannabinoids in keratinized matrices.
The quantifier MRM transitions for each of the target analytes were used to generate calibration curves. Figure 3 displays resulting regression lines plotted across the calibrator levels for:
- Androstenedione 13C3, cortisone 13C3, and progesterone 13C3 from a to 500 pg/mg
- 2-AG D5 from 5 to 200 pg/mg
- N-OEA D4 and PEA D4 from 500 to 100000 pg/mg
- AEA D4 and testosterone 13C3 from 0.1 to 10 pg/mg extracted from hair samples
Calibration curves generated via this approach exhibited excellent linearity across the calibration ranges with R2 values higher than 0.99 for every targeted steroid hormone and endocannabinoid. Outstanding linearity was also noted in terms of the analytes extracted from nail samples (data not shown).
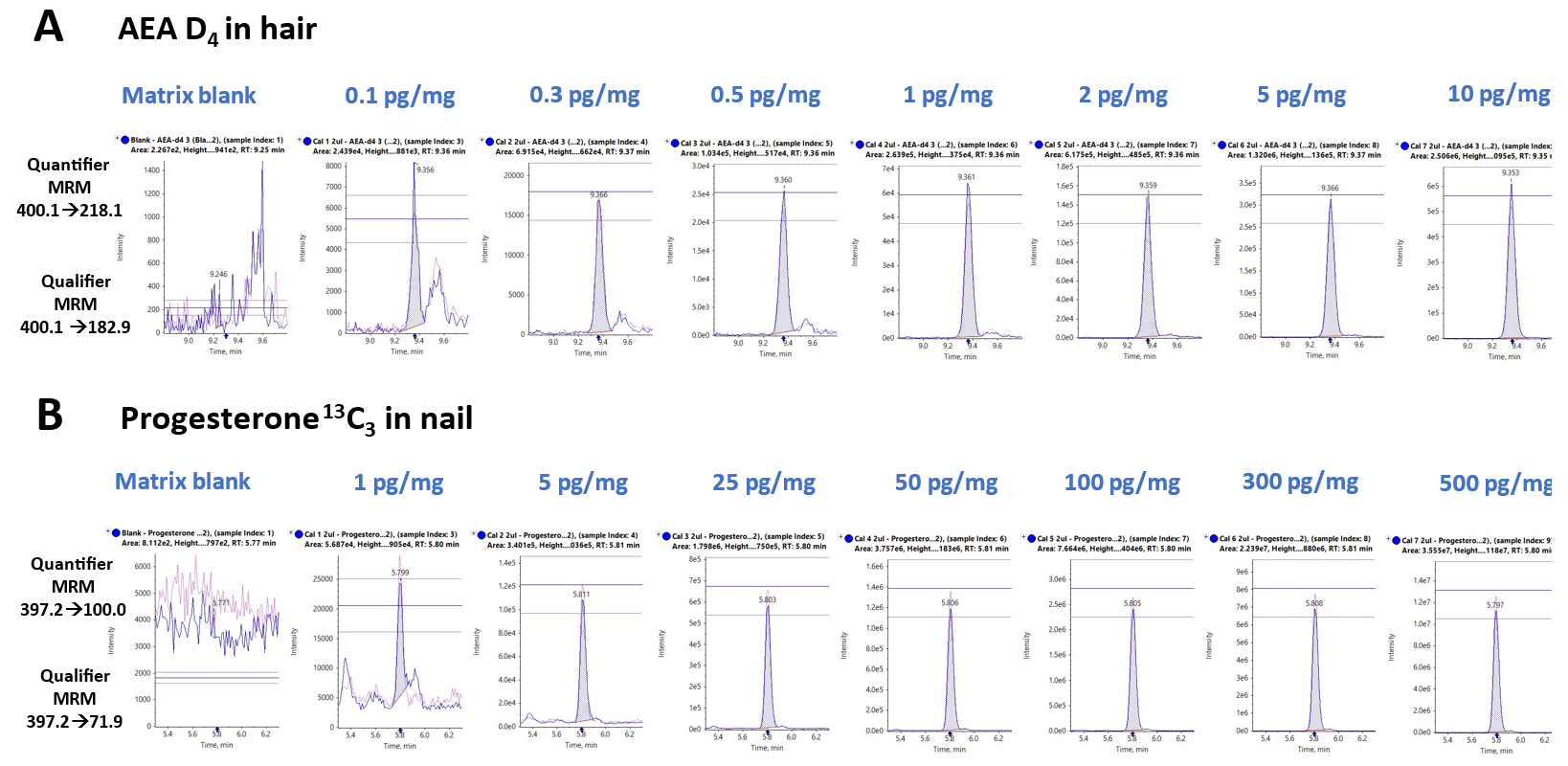
Figure 2. Representative extracted ion chromatograms (XICs) for A) a heavy labeled endocannabinoid analog extracted from hair samples and B) a heavy labeled steroid hormone analog extracted from nail samples. XICs for A) AEA-D4 from 0.1 to 10 pg/mg and B) progesterone 12C3 from 1 to 500 pg/mg. Both the quantifier and qualifier traces are shown. Ion ratios were also monitored across the dataset as shown by the tolerance lines. Image Credit: SCIEX
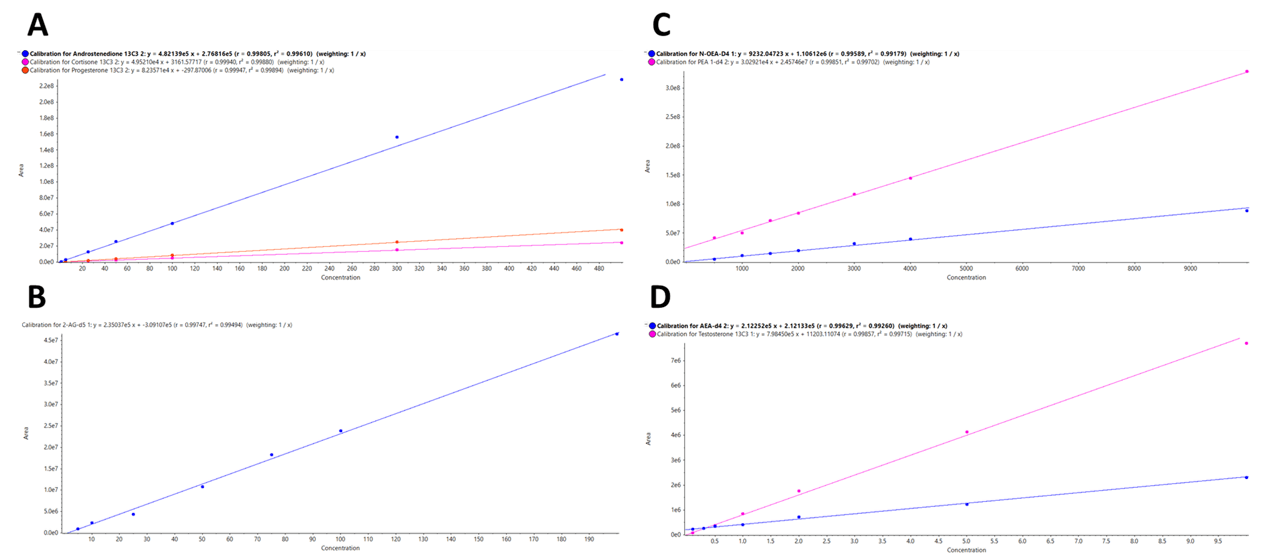
Figure 3. Excellent linearity for the 8 steroids and endocannabinoids targeted in this panel. Calibration curves generated using the quantifier MRM transition for A) androstenedione 13C3, cortisone 13C3, and progesterone 13C3 from a to 500 pg/mg, B) 2-AG D5 from 5 to 200 pg/mg, C) N-OEA D4 and PEA D4 from 500 to 100,000 pg/mg and D) AEA D4 and testosterone 13C3 from 0.1 to 10 pg/mg extracted from hair samples. The assay showed excellent linearity with R2 values greater than 0.99 for all the analytes. Image Credit: SCIEX
Leveraging sensitivity improvement for low-level detection of endogenous steroids and endocannabinoids in hair and nail samples
Sensitive mass spectrometry instrumentation is a key tool in accurately quantifying low-level endogenous species found within complex biological matrices.
This study compared the SCIEX 7500 system’s sensitivity to that of a previous-generation instrument, the QTRAP 6500+ system, using both the signal-to-noise ratio and peak area.
Figure 4 features the XIC series for androstenedione 13C3 in hair samples obtained via the SCIEX 7500 system (top) and QTRAP 6500+ system (bottom) at concentration levels ranging from 1 to 500 pg/mg. The respective peak area and SIGNAL-TO-NOISE values are also shown.
Average peak area and SIGNAL-TO-NOISE gains across the seven concentration levels for androstenedione 13C3 were determined to be 9.85 ±1.21 and 4.39 ±2.20, respectively.
The other seven analytes included in the panel exhibited similar results, with peak area gains in hair samples ranging from 9.85x for androstenedione 13C3 to 71.21x for PEA D4 and SIGNAL-TO-NOISE gains ranging from 0.62x for progesterone 13C3 to 13.82x for PEA D4, respectively.
It was noted that the SCIEX 7500 system substantially increased the peak areas and signal-to-noise ratios across all the analytes extracted from hair samples. Average peak area gains of 29.95x and average SIGNAL-TO-NOISE gains of 5.60x were calculated across all the analytes extracted from hair samples.
Figure 5 displays the XIC series for 2-AG-D5 in nail samples obtained using the SCIEX 7500 system (top) and QTRAP 6500+ system (bottom) for seven different concentration levels ranging from 5 to 200 pg/mg.
Average peak area and SIGNAL-TO-NOISE gains across the seven concentration levels for 2-AG-D5 was determined to be 35.49 ±4.75 and 4.48 ±4.04, respectively. Significant gains in the peak area and SIGNAL-TO-NOISE were observed for the analytes extracted from nail samples, similar to those observed from hair samples.
Peak area gains were found to range from 3.69x for progesterone 13C3 to 42.55x for PEA D4 while SIGNAL-TO-NOISE gains were found to range from 0.68x for progesterone 13C3 to 19.04x for PEA D4 in nail samples, respectively.
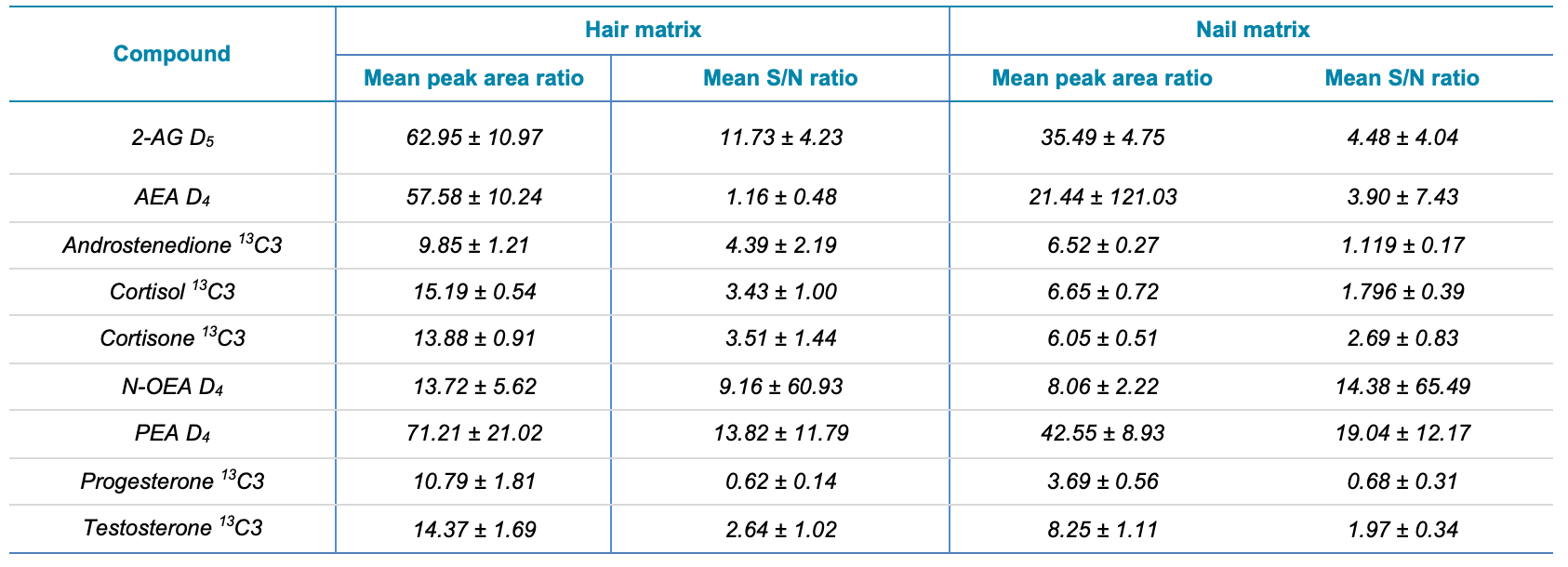
Overall, peak area gains and SIGNAL-TO-NOISE gains were found to average 15.41x and 5.56x, respectively, across all analytes extracted from nail samples. Table 1 provides a useful summary of peak area and SIGNAL-TO-NOISE gains observed for the eight target analytes in the two keratinized matrices investigated.
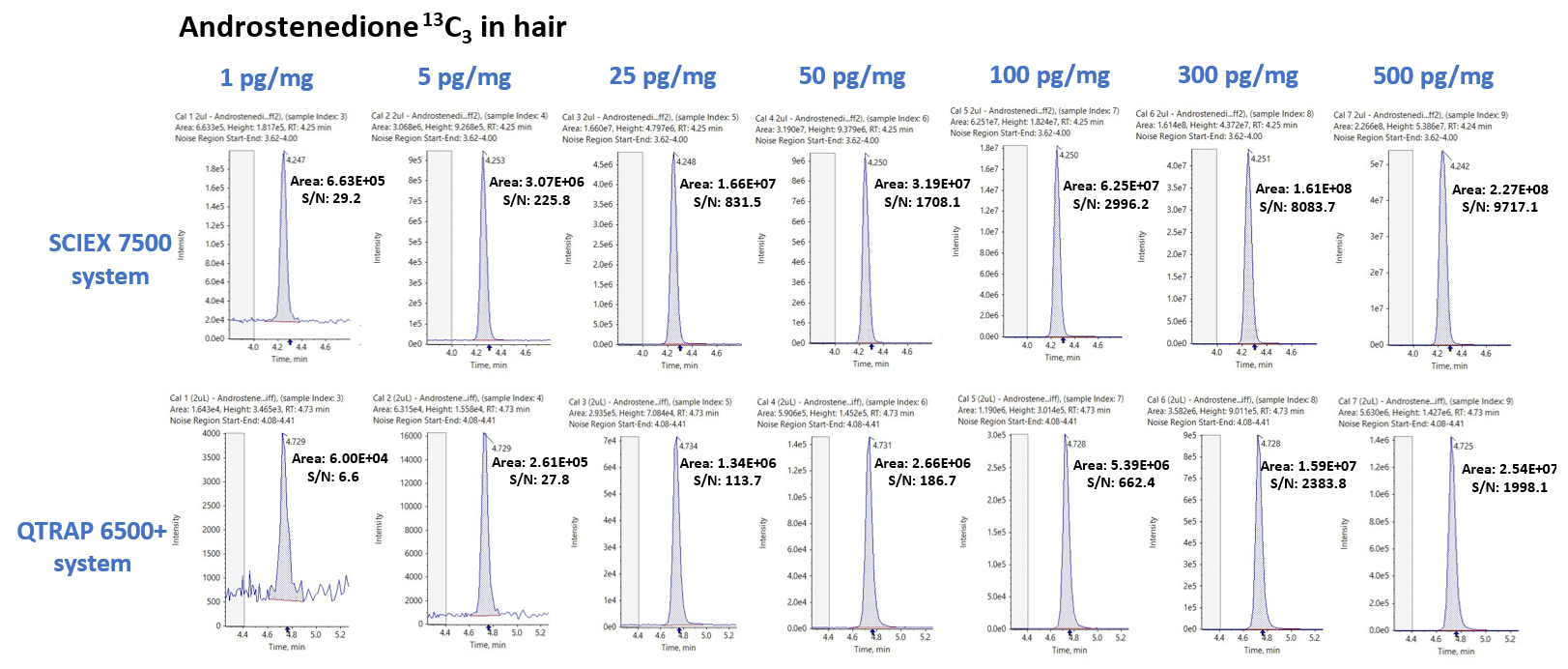
Figure 4. Sensitivity improvement for the detection of steroid hormones extracted from hair samples. Extracted ion chromatogram (XIC) comparisons between the SCIEX 7500 system (top) and the QTRAP 6500+ system (bottom) for the seven levels of calibrators of androstenedione 13C3 ranging from 1 to 500 pg/mg extracted from hair samples. The SCIEX 7500 system showed significant improvements in both peak area and S/N gains over the QTRAP 6500+ system. Image Credit: SCIEX
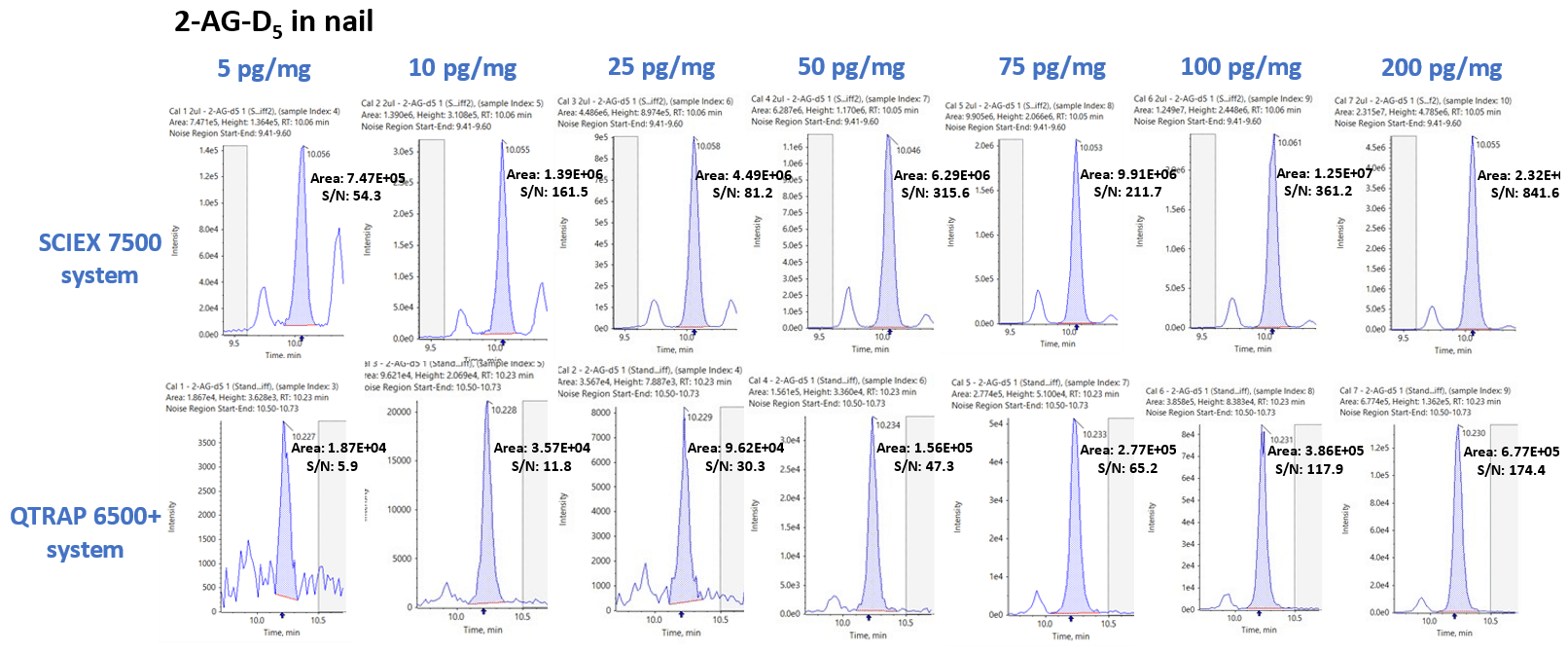
Figure 5. Increased sensitivity for the detection of endocannabinoids extracted from nail samples. Extracted ion chromatogram (XIC) comparisons between the SCIEX 7500 system (top) and the QTRAP 6500+ system (bottom) for the seven levels of calibrators of 2-AG-D5 ranging from 5 to 200 pg/mg extracted from nail samples. The SCIEX 7500 system showed significant improvements in both peak area and S/N gains over the QTRAP 6500+ system. Image Credit: SCIEX
Table 1. Mean peak area and S/N ratios of the seven levels of calibrators for each of the 9 analytes spiked in hair and nail samples. Seven levels of calibrators were prepared and injected to the SCIEX 7500 system and QTRAP 6500+ system. The ratios of the peak areas and S/N were determined at each calibrator level and averaged for each of the 5 steroid hormones and 4 endocannabinoids for each of the two keratinized matrices analyzed (hair and nail). Source: SCIEX
Conclusions
The study presented here demonstrated a sensitive, reliable analytical workflow suitable for the quantification of steroid hormones and endocannabinoids in human hair and nail matrices.
The study leveraged the SCIEX 7500 system’s technological improvements to enhance sensitivity, enabling the accurate detection of endogenous markers present in keratinized matrices.
Ion ratio difference was determined to be <20% for the target analytes’ quantifier and qualifier ions, illustrating the workflow’s quantitative robustness.
Overall, the system's linearity was excellent, with R2 values >0.99 for all targeted steroids and endocannabinoids.
The signals observed on the SCIEX 7500 system and the previous generation QTRAP 6500+ system were compared. This helped assess the impact of these sensitivity gains. There were noteworthy improvements in terms of the peak area and signal-to-noise gains for the eight target analytes in the two keratinized matrices investigated.
For the compounds extracted from hair and nail samples, respectively, average peak area gains ranged from 9.85x to 71.21x and 3.69x to 42.55x. It was also determined that the average signal-to-noise ratio gains for compounds extracted from hair and nail samples ranged from 0.62x to 13.82x and 0.68x to 19.04x, respectively.
The capacity of the SCIEX 7500 system to robustly and routinely detect ultra-low levels of analytes extracted from challenging biological matrices was showcased throughout this study. The workflow described offers the level of sensitivity required for long-term retrospective measurement of endogenous biomarkers in keratinized matrices.
References and further reading
- Voegel CD, Baumgartner MR, Kraemer T, Wüst S, Binx TM (2021) “Simultaneous quantification of steroid hormones and endocannabinoids (ECs) in human hair using an automated supported liquid extraction (SLE) and LC-MS/MS - Insights into EC baseline values and correlation to steroid concentrations”, Talanta, 222:121499.
Acknowledgments
Produced from materials originally authored by Clarissa D. Voegel, Patrick Graefling, and Tina M. Binz, from the Center for Forensic Hair Analytics, Zurich Institute of Forensic Medicine, University of Zurich, Zurich, Switzerland; Maja E. Keller and Thomas Kraemer from the Department of Forensic Pharmacology and Toxicology, Zurich Institute of Forensic Medicine, University of Zurich, Zurich, Switzerland; and Pierre Negri from SCIEX, USA.
About SCIEX
SCIEX mission is to deliver solutions for the precision detection and quantitation of molecules, empowering their customers to protect and advance the wellness and safety of all.
SCIEX has led the field of mass spectrometry for 50 years. From the moment we launched the first ever commercially successful triple quad in 1981, they have developed groundbreaking technologies and solutions that influence life-changing research and outcomes.
Today, as part of the Danaher family of global life science and technology innovators, they continue to pioneer robust solutions in mass spectrometry and capillary electrophoresis. But they don’t just develop products. It is what they do together with their customers that sets them apart. That’s why thousands of life science experts around the world choose SCIEX to get the answers they can trust to better inform critical decisions. Decisions that positively impact lives.
They proudly stand behind our tagline: The Power of Precision.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.