Using the Liver-on-a-chip hepatic co-culture model (assessed by CN-Bio’s partners at the US FDA), the DILI in vitro Service can screen the gene editing reagents, antibodies, small molecules, and ASO to determine the risk of DILI in humans.
Service overview
At least six hepatic health parameters, including clinical indicators of liver injury, are simultaneously assessed by the service. This method attains the necessary sensitivity and specificity to detect hepatotoxins that animals fail to detect, along with other in vitro tests.
- Check for CYP induction or inhibition
- Bespoke multi-organ, or studies with circulating immune cells available on request
- Test a range of drugs to enable lead or candidate selection
- Investigate drug-drug interaction events
- Derive human data to complement animal studies and explain non-concordant findings.
- Use retained samples to understand drug metabolism
- Explore DILI under a range of conditions: healthy, inflammation, fatty
- Test highly human-specific new modalities
- Compare acute vs. chronic toxicity responses
Customer feedback
I worked with CN-Bio on a DILI Services project to predict which formulation of the same test agent would be safe for humans. Previous In vivo studies demonstrated inter-species differences between the formulations in animals but thanks to the expertise of CN-Bio, I was able to rapidly gain human-relevant data that helped to move my project forward.”
Gerry Boss MD, Distinguished Professor of Medicine, Department of Medicine, University of California, San Diego
How the service works
The assay comprehensively evaluates DILI risk by co-culturing primary human hepatocytes and non-parenchymal cells and assessing multiple endpoints.
This service offers enough throughput to deliver concentration-response curves and higher content data than spheroids.
The dedicated contact will collaborate with users from the beginning to the finish of the project.
- One to two months to complete the study from receiving an order
- Two weeks to run endpoint assays, analyze data, and complete the report
- Design and finalize the experiment plan
- Customer supplies the required amount of drug(s)
- Two weeks to complete cell culture
Standard DILI cell culture timeline
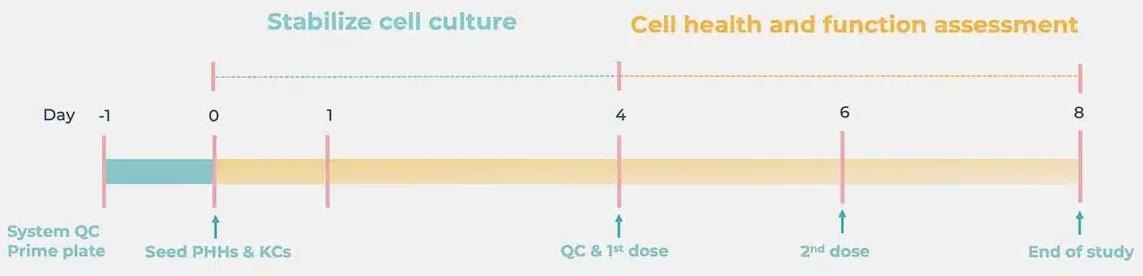
Image Credit: CN-Bio
Endpoint measurements
Image Credit: CN-Bio
Included, but are not limited to:
Functionality biomarkers
- Urea production
- Cytochrome P450 enzyme activity
- Albumin production
Clinical liver health biomarkers
- Aspartate Transferase (AST)/Alanine amino transferase (ALT)
- Lactose dehydrogenase (LDH) release
- Adenosine Triphosphate (ATP)
Optional profiling analysis
- Transcriptomics
- Quantitative PCR
Related applications
Drug-induced liver injury
Image Credit: CN-Bio
The DILI test can determine the toxicity of various entities (small molecules, proteins, ASOs, AAVs, etc.) in the presence or absence of liver disease.
It enables better-informed predictions of human liver responses to acute and chronic drug exposure by employing a wide range of clinically translatable endpoints.