With the ability to replicate important disease phenotypes like fibrosis and to report clinical biomarkers that enable translatability to clinical trials, Non-Alcoholic steatohepatitis (NASH), also known as Metabolic dysfunction-associated steatohepatitis (MASH), in vitro services provide a preclinical tool for testing therapeutics in a human-relevant model.
NASH in vitro services overview:
CN-Bio takes a collaborative approach, leveraging its broad NASH knowledge to build an experiment that best meets the needs of researchers. The proven PhysioMimix NASH assay utilizes CN Bio's tri-culture liver-on-a-chip model comprised of primary human hepatocytes, Kupffer and stellate cells. It can be tailored to answer particular queries, focus on alternative endpoints, or give samples for -omics research.
- Examine biomarkers for cell health, fibrosis, steatosis, and inflammation
- Identify clinical markers that can be translated
- Examine or verify the impact of your treatment using a model approved by the industry.
- Complete the project report, including all raw data, methods, and results
- Employs verified cells with known genotypes associated with NASH
- The choice to include sample -omics analysis
Customer feedback
Working with CN-Bio has been a pleasure, the team listened to our non-trivial requirements and made useful suggestions to help meet objectives. During the course of the project, the team were responsive and helpful. The results were delivered as agreed and helped us to move towards our goals.”
Dr. Giuseppe Ferrandino, Senior Translational Scientist, Owlstone Medical
Heather Hsu, Chief Scientific Officer at Inipharm stated, “Inipharm is developing a drug that targets a genetic metabolic target which has metabolomics differences between mice and men. For this reason, we evaluated several different primary human testing systems that have features of NASH. We found CN- Bio has developed a system that has steatotic, inflammatory, and fibrotic features that have been very useful in evaluating our lead compounds. The scientists at CN-Bio are very knowledgeable and have worked with us on the study details to obtain the best quality of results in a timely manner.”
How the service works:
The assay provides a comprehensive evaluation of the efficacy of NASH drugs by co-culturing primary human hepatocytes and non-parenchymal cells and assessing several endpoints.
As an adjunct to murine models, this service produces human-relevant data that encapsulates the essential pathophysiology of NASH.
The dedicated contact will collaborate with users from the beginning to the end of the project.
- Design and finalize the experiment plan
- The customer supplies the required amount of drug(s)
- Cell culture takes three to four weeks to finish
- Running endpoint tests, analyzing data, and finishing the report will take two to three weeks
- After getting an order, the research takes around two months to finish
Standard NASH cell culture timeline

Image Credit: CN-Bio
Endpoint measurements
Image Credit: CN-Bio
Included, but are not limited to:
Functionality biomarkers
- Albumin production
- Urea production
Clinical liver health biomarkers
- Lactose dehydrogenase (LDH) release
- Aspartate Transferase (AST)/Alanine amino transferase (ALT)
Disease biomarkers
- Luminex®/ELISA assays
- Fibrosis (e.g. TIMP-1, Pro-collagen, Fibronectin)
- Inflammation (e.g. IL-6, IL-8 TNF-α)
- Confocal microscopy
- Smooth muscle actin
- Collagen
- Fat accumulation (Nile Red staining)
Related applications
Non-alcoholic steatohepatitis
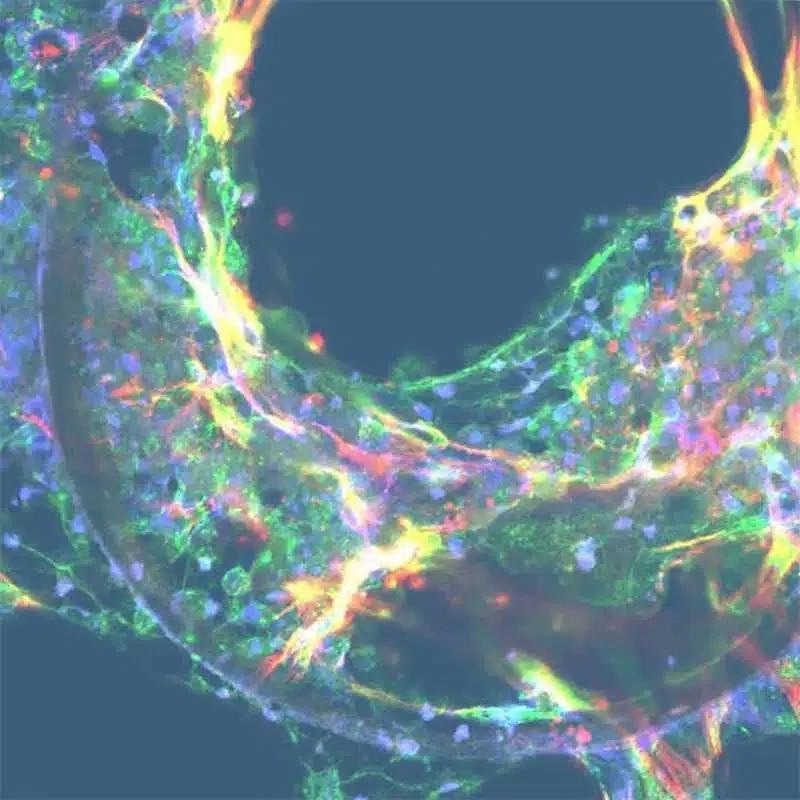
Image Credit: CN-Bio
Anti-NASH treatments have not proven effective in clinical trials; with only one market-approved therapeutic available. A novel preclinical test is needed to enhance human efficacy predictions. The PhysioMimix NASH assay offers a pre-clinical in vitro model that perfectly replicates the vital characteristics of this prevalent liver disease utilizing human tissue.