Reduce the workload associated with cell validation ahead of Organ-on-a-chip assays to hasten the transmission of human translatable data.
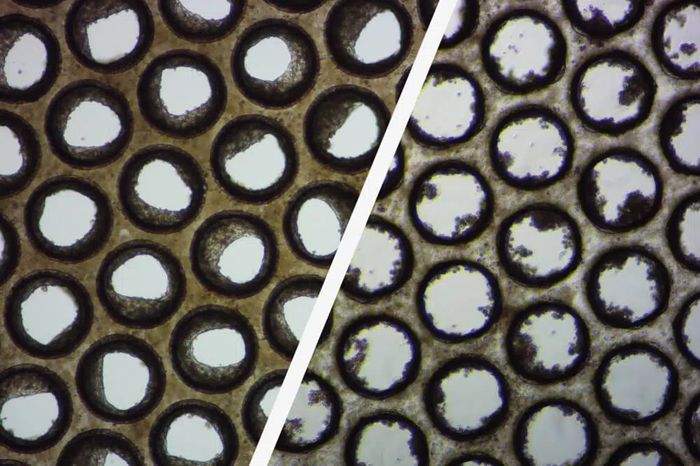
Good/poor liver microtissue. Image Credit: CN-Bio
Pre-validation of primary cells ahead of Organ-on-a-chip (OOC) studies is often disregarded despite being essential to the assay's success. Negative microtissue formation, subpar assay performance, and erroneous data result from using unvalidated cells. Though expensive and time-consuming, the validation process is essential.
Obtaining two verified hepatocyte lots for OOC tests costs around $15K in materials and 100+ hours of a professional scientist’s effort.
This burden is removed from the user’s laboratory workflow with a portfolio of 3D-validated cells. Select an option from the catalog to expedite the replication of PhysioMimix models.
3D-validated cell features
- Promised to maintain function and phenotype for two weeks in the culture
- Verified in the applications section for both mono and co-culture assays
- There are numerous individual donors accessible
- PhysioMimix systems and SOPs have been tested for compatibility
- Vials are widely available for each donor
- Verified tissue function, metabolic activity, and three-dimensional growth
Benefits of using 3D-validated cells
- Concentrate the user’s time on experiments that add value
- Eliminates the burden of in-house quality control testing with accessible, off-the-shelf access
- Use cells that have been shown to thrive to make the transition to OOC easier
- Provides consistent inter-assay performance for data assurance
Currently available 3D-validated cells
Source: CN-Bio
| |
Source |
Cells/vial |
Order from |
| Primary human hepatocyte cells |
LifeNet Health LifeSciences |
min 5M/vial |
LifeNet Health LifeSciences |
| Primary human Kupffer cells |
LifeNet Health LifeSciences |
min 500K/vial |
LifeNet Health LifeSciences |
| Primary human hepatocyte cells |
Lonza |
min 5M/vial |
CN-Bio |
| Primary human Kupffer cells |
Lonza |
min 500K/vial |
CN-Bio |
Hepatocyte thawing medium
Hepatocyte thawing medium is not supplied and must be sourced from the appropriate manufacturer to optimize post-thaw hepatocyte production.
The effect of poor lot quality
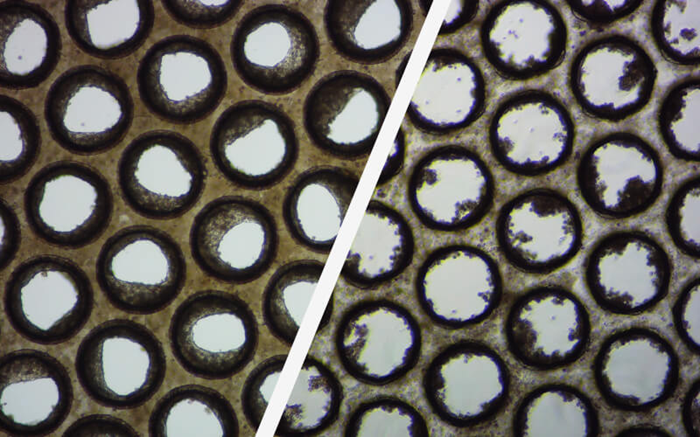
Image Credit: CN-Bio
Over 60% of the primary human hepatocyte batches the experts have examined over the past five years have not formed liver microtissues or performed within acceptable limits in OOC tests.
These brightfield pictures show how the pores of individual Multi-chip Liver-12 plate scaffolds form good (left) and poor (right) liver microtissue formation.
The effect of donor quality over 14 days on microtissue functionality (albumin production) is highlighted in the graph. By utilizing 3D-validated cells, the possibility of subpar assay performance is eliminated.
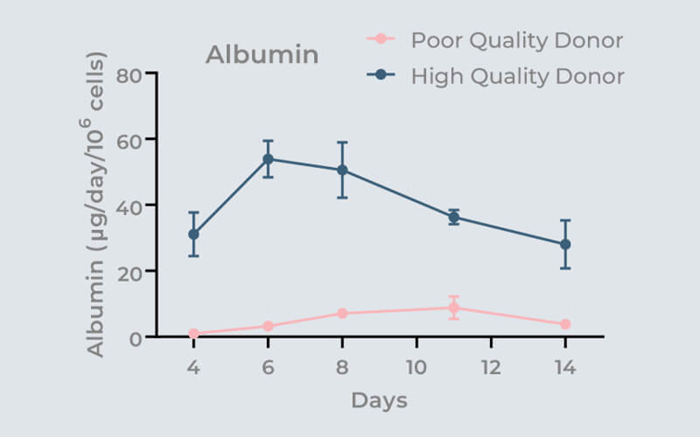
Image Credit: CN-Bio
Validation reports
The lengthy procedure of verifying donor cells for use in OOC tests is detailed in the sample reports. The quality control protocols guarantee the formation of stable, highly functioning three-dimensional liver microtissues from mono-or co-cultures.

Image Credit: CN-Bio
Supported applications
To meet various application requirements, 3D-verified cells can be obtained for mono- or duo-culture. Tri-cultures are available as NASH-in-a-box kits.
Source: CN-Bio
| Liver model |
Cell type |
Applications |
Required products |
| Mono-culture |
Primary human hepatocytes |
HBV, Drug metabolism |
PhysioMimix, Liver plates, 3D validated cells |
| Duo-culture |
Primary human hepatocytes + Kupffer Cells |
HBV, DILI, Immune-mediated toxicity |
PhysioMimix, Liver plates, 3D validated cells |
| Tri-culture |
Primary human hepatocytes + Kupffer Cells + Stellate Cells |
NASH |
PhysioMimix, NASH-in-a-box |