Lipid nanoparticles (LNPs) are the carrier vehicles that protect messenger RNA (mRNA) molecules from degradation and aid intracellular delivery and endosomal escape in the nucleoside-modified mRNA-LNP vaccine platforms currently used by Pfizer/BioNTech and Moderna, against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These two vaccines surpass the rest with their effective support for T follicular helper cells (Tfh) and the protective humoral responses observed in the preclinical trials.
While the vital role of LNPs in these vaccines' action is established, the potentially inflammatory nature of these LNPs is not assessed. Also, in the Pfizer/BioNTech and Moderna vaccines' human trials, it is reported that side effects often linked to inflammation, such as pain, swelling, fever, and sleepiness, are common.
Because the vaccine was presumed to be non-inflammatory, these side-effects were taken to be generated from the potent immune response to the vaccine. Therefore, there is a need for a systemic approach to analyze the inflammatory properties of LNPs and understand their role in the vaccination process.
In a recent bioRxiv* preprint research paper, Botond Z. Igyártó and colleagues from Thomas Jefferson University demonstrated the inflammatory nature of the lipid nanoparticles (LNPs). Upon intradermal administration in mice, they observed massive neutrophil infiltration, activation of diverse inflammatory pathways, and production of various inflammatory cytokines and chemokines. They observed similar observations when the LNPs were delivered intranasally.
Notably, intranasal inoculation with the same dose (10 μg) of LNP (as in the intradermal delivery) caused a high mortality rate in mice – about 80% of the treated mice died in less than 24 hours. The researchers found that the LNPs' inflammatory properties are not site-specific. They observed a fast diffusion, dispersion, and distribution rate in the tissues, upon internal delivery.
"Thus, the inflammatory milieu induced by the LNPs could be partially responsible for reported side effects of mRNA-LNP-based SARS-CoV-2 vaccines in humans, and are possibly contributory to their reported high potency for eliciting protective immunity."
The LNPs consist of a mixture of phospholipids, cholesterol, PEGylated lipids, and cationic or ionizable lipids. These improve structure, stability and support prolonged circulation. The cationic/ionizable lipids, complex with the negatively charged mRNA molecules and enable the exit of the mRNA from the endosome to the cytosol for translation.
It is reported that some LNPs containing ionizable/cationic lipids are highly inflammatory and possibly cytotoxic. A preclinical study showed adjuvant activity of mRNA complexed with LNPs. To avoid activation of innate inflammatory pathways, in the vaccine, the mRNA is nucleoside-modified.
In this study, the researchers noted that the inflammatory nature of the LNPs could explain their potent adjuvant activity and their superiority, compared to other adjuvants, in supporting the induction of adaptive immune responses.
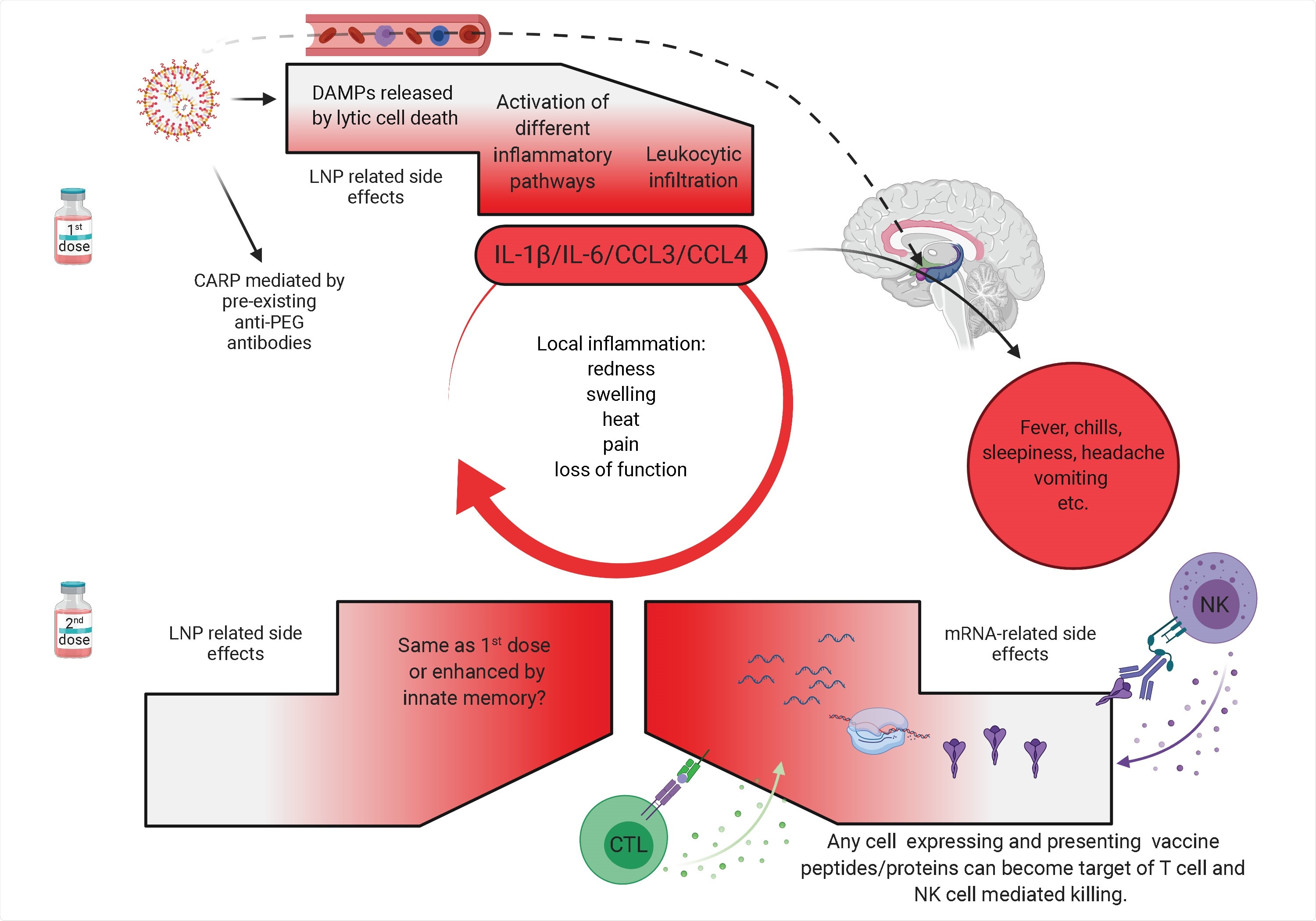
Potential mechanism of side effects. The side effects observed with the SARS-CoV-2 vaccine’s first dose are likely associated with the LNPs’ inflammatory properties. LNPs activate different inflammatory pathways that will lead to the production of inflammatory cytokines, such as IL-1β and IL-6 that can initiate and sustain local and systemic inflammations and side effects. LNPs might also diffuse from the periphery and reach any organs in the body, including CNS (hypothalamus) where they could directly induce side effects (dashed line). PEG is widely used as a food and medicine additive, and many of us develop antibodies to PEG. Therefore, the LNPs’ PEGylated lipids can induce CARP in humans with preexisting PEG-specific antibodies. Humans often experience more severe side effects with the second dose. Here we posit that might be due to multiple reasons. Firstly, innate immune memory against the LNPs might form after the first vaccination, and that could lead to even more robust inflammatory responses upon the second vaccination. Secondly, after the first vaccination adaptive immune responses are formed targeting the viral protein coded by the mRNA. As such, cells (shown as red shape) expressing the viral protein derived peptides or protein itself can become the target of CD8+ T or NK cell-mediated killing (ADCC), respectively. Since the LNPs could diffuse throughout the body and transfect any cell in their path with the mRNA, and the mRNA could also be further distributed through extracellular vesicles (Maugeri et al., 2019), the target population could potentially be vast and diverse.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
As expected with the observed side-effects in humans after intramuscular vaccination with the Pfizer/BioNTech or Moderna vaccines the intradermal inoculation of LNPs in mice led to secretion of the pyrogens - IL-1β/IL-6 and macrophage inflammatory protein-α (CCL3) and macrophage inflammatory protein-β (CCL4).
The researchers conducted Gene Set Enrichment Analysis (GSEA) to understand the pathways involved in the inflammatory cascade with the proprietary LNPs administered. The observed activated TLR pathways, among others, and upregulated inflammasome components such as Nlrp3 and enrichment of genes involved in necroptosis. In concordance, flow cytometry data revealed significant inflammatory cell death (after inoculation with LNPs), such as necroptosis and pyroptosis.
"Overall, the robust inflammatory milieu induced by LNPs, combined with the presentation of the vaccine-derived peptides/protein outside of antigen-presenting cells, might cause tissue damage and exacerbate side effects."
The researchers raised a concern that the LNPs, as lipid particles that can diffuse quickly, could potentially gain access to the central nervous system (CNS) through the olfactory bulb or blood. However, this needs to be determined with further study. Also, the role of innate memory responses to LNPs needs to be studied.
In conclusion, the intradermal inoculation with LNPs induced robust inflammation in mice. Using different techniques, the researchers showed that the LNPs, alone or complexed with control non-coding poly-cytosine mRNA, are highly inflammatory in mice - likely through the engagement and activation of various distinct and convergent inflammatory pathways.
Importantly, this study showed that the intradermal or intranasal delivery in mice of LNPs used in preclinical studies triggers inflammation characterized by leukocytic infiltration, activation of different inflammatory pathways and secretion of a diverse pool of inflammatory cytokines and chemokines.
However, further studies will be needed to determine the exact nature of the inflammatory responses triggered by mRNA-LNP vaccines in humans and how much overlap there might be with the inflammatory signatures documented here for mice, the researchers write.
"However, it will be necessary to strike a balance between positive adjuvant and negative inflammatory properties as LNP-associated vaccines move forward."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory, Sonia Ndeupen, Zhen Qin, Sonya Jacobsen, Henri Estanbouli, Aurélie Bouteau, Botond Z. Igyártó, bioRxiv 2021.03.04.430128; doi: https://doi.org/10.1101/2021.03.04.430128, https://www.biorxiv.org/content/10.1101/2021.03.04.430128v1
- Peer reviewed and published scientific report.
Ndeupen, Sonia, Zhen Qin, Sonya Jacobsen, Aurélie Bouteau, Henri Estanbouli, and Botond Z. Igyártó. 2021. “The MRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory.” IScience 24 (12): 103479. https://doi.org/10.1016/j.isci.2021.103479. https://www.cell.com/iscience/fulltext/S2589-0042(21)01450-4.