Scientists from the Cleveland Clinic, USA, have recently evaluated the effectiveness of coronavirus disease 2019 COVID-19) vaccination among individuals with or without a history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
The study findings reveal that individuals with previous SARS-CoV-2 infection do not get additional benefits from vaccination, indicating that COVID-19 vaccines should be prioritized to individuals without prior infection. The study is currently available on the medRxiv* preprint server (not peer-reviewed).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
In the United States, the US Food and Drug Administration (FDA) has provided emergency use authorization for two mRNA-based COVID-19 vaccines developed by Pfizer/BioNTech and Moderna, which have shown high efficacy against SARS-CoV-2 infection and COVID-19 disease in clinical trials. However, the ability to vaccinate a large part of the global population is limited by vaccine supply.
In order to ensure fair access to vaccines throughout the world, the COVID-19 vaccines Global Access (COVAX) initiative was launched. In many countries, especially those with low socioeconomic status, there is a serious shortage of vaccines. Thus, in order to get the maximum vaccine benefits, the most vulnerable population should be prioritized for the vaccination.
Currently, most countries prioritize vaccination for healthcare and other frontline workers, elderly people, and people with comorbidities.
To further narrow down the prioritization criteria, the scientists in the current study have evaluated the necessity of COVID-19 vaccines for individuals who were previously infected with SARS-CoV-2.
Study design
The study was conducted on 52,238 employees in the Cleveland Clinic. A positive RT-PCR test was considered to define SARS-CoV-2 infection. The participants received two doses of the Pfizer/BioNTech or Moderna COVID-19 vaccine at an interval of 28 days. A participant was considered vaccinated after 14 days of receiving the 2nd vaccine dose. Similarly, a participant who tested positive for SARS-CoV-2 at least 42 days before the vaccination initiation was considered previously infected.
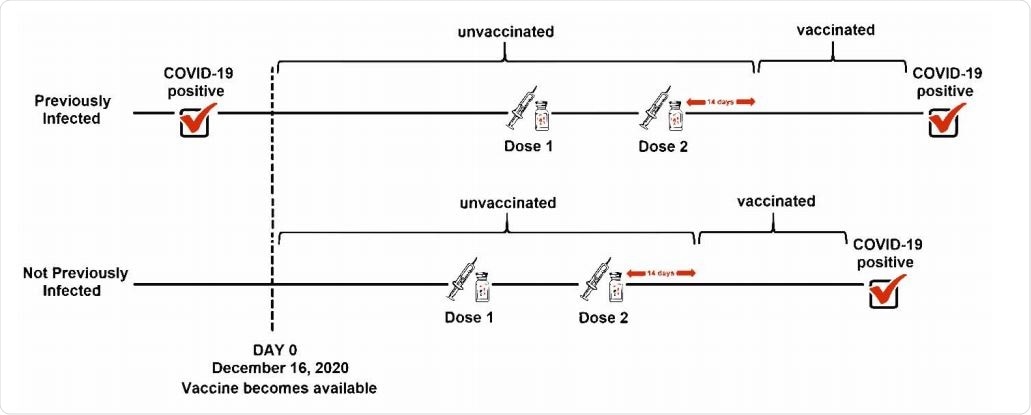
Explanation of “previously infected” analyzed as a time-independent covariate and “vaccinated” treated as a time-dependent covariate.
Important observations
Of all enrolled participants, 5% had previous SARS-CoV-2 infection. Compared to 59% of non-infected participants, only 47% of previously infected participants were vaccinated by the end of the study. About 63% of all vaccinated participants received the Moderna vaccine.
The analysis of cumulative COVID-19 incidence revealed that during the course of the study, SARS-CoV-2 infection occurred almost exclusively in participants who were not previously infected and were not vaccinated.
Interestingly, no significant difference in COVID-19 incidence was observed between previously infected and currently unvaccinated participants, previously infected and currently vaccinated participants, and previously uninfected and currently vaccinated participants.
The participants from these three groups exhibited a significantly lower incidence of SARS-CoV-2 infection compared to previously uninfected and currently unvaccinated participants.
Specifically, of all infections during the study period, 99.3% occurred in participants who were not infected previously and remained unvaccinated. In contrast, only 0.7% of infections occurred in participants who were not previously infected but were currently vaccinated.
Importantly, not a single incidence of SARS-CoV-2 infection was observed in previously infected participants with or without vaccination.
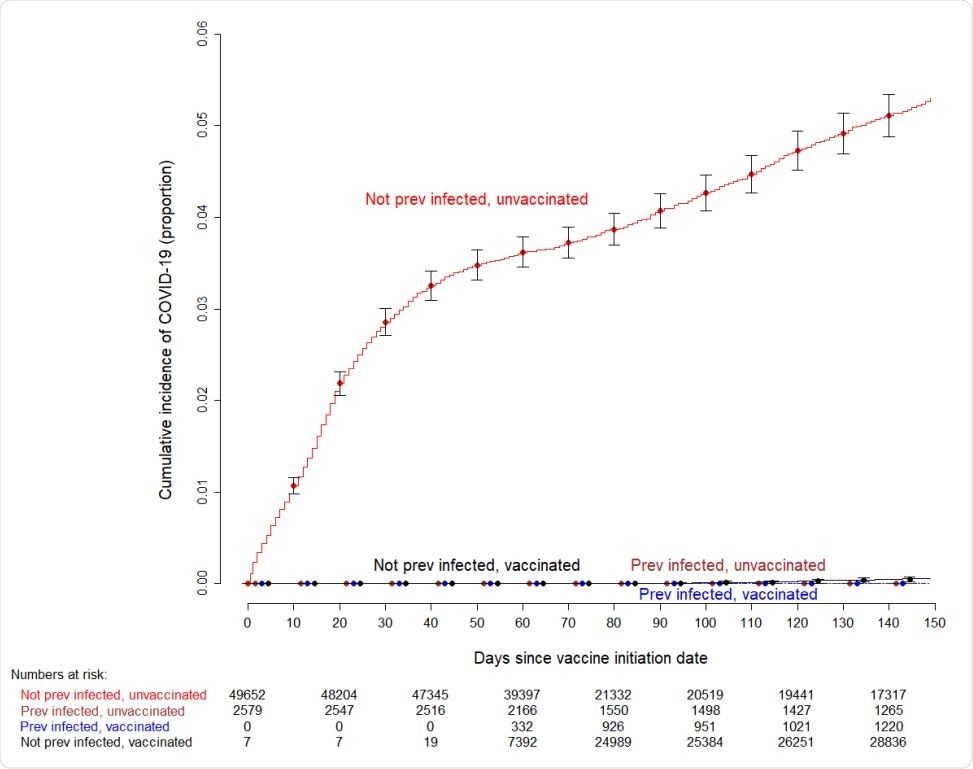
Simon-Makuch plot showing the cumulative incidence of COVID-19 among subjects previously infected and not previously infected with COVID-19, who did and did not receive the vaccine. Curves for the unvaccinated are based on data for those who did not receive the vaccine during the duration of the study, and for those waiting to receive the vaccine. Day zero was Dec 16, 2020, the day vaccination was started in our institution. Error bars represent 95% confidence intervals. Seven subjects who had been vaccinated earlier as participants in clinical trials were considered vaccinated throughout the duration of the study. Twelve subjects who received their first dose in the first week of the vaccination campaign managed to get their second dose three weeks later, and were thus considered vaccinated earlier than 42 days since the start of the vaccination campaign
With further statistical analysis, it was observed that the COVID-19 vaccination significantly reduced the risk of SARS-CoV-2 infection in previously uninfected participants but not in previously infected participants.
Although the study did not directly estimate the duration of protection from natural infection, it was observed that previously infected participants remained protected against COVID-19 for at least 10 months after the symptom onset or a positive test result.
Study significance
The scarcity of vaccines, coupled with the knowledge that vaccines do not provide additional protection to those who have already been infected, is the strongest argument for restricting vaccine administration to those who have not had the infection.
In addition to the profession, age, and comorbid conditions, previous infection should be an important consideration in deciding whom to prioritize to receive the vaccine.
A practical and useful message would be to consider symptomatic COVID-19 to be as good as having received a vaccine, and that people who have had COVID-19 confirmed by a reliable laboratory test do not need the vaccine.
The study concludes, "individuals who have laboratory-confirmed symptomatic SARS-CoV-2 infection are unlikely to benefit from COVID-19 vaccination, and vaccines can be safely prioritized to those who have not been infected before."
In contrast, individuals without prior SARS-CoV-2 infection can get the maximum benefits from vaccination. Thus, based on the study findings, COVID-19 vaccines should be prioritized to naïve individuals without a history of SARS-CoV-2 infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources