Dec 7 2015
Researchers unravel abilities of the immune system’s giant cells
If rubbish is too big and unwieldy for normal household waste, its removal becomes the job of specialized experts. Researchers from the Technical University of Munich (TUM) have now discovered, in cooperation with colleagues from the UK, how large, fused cells help our body to deal with bulky items that may otherwise obstruct normal physiological processes. While scientists have long known about these giant cells, a clear picture of their abilities was still lacking. The current studies, published in the December issue of Cell Reports, explain why a new treatment for systemic amyloidosis, currently undergoing clinical trials, may be so effective.
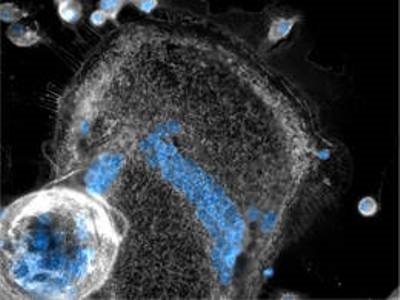
Microscopic picture of giant cells MGCs and macrophages stained with membrane-specific (grey) and nucleus-specific (blue) dyes. One large adherent (center) and one sphere-shaped semi-adherent (lower left corner) MGC are surrounded by a number of mononucleated macrophages. (Picture: Ronny Milde / TUM)
Pathologists have known about the existence of multinucleated giant cells (MGCs) for over a century. The cells occur in certain chronic inflammatory diseases, such as tuberculosis, but also during the foreign body reaction to implants, such as an artificial hip. Still, systematic research into the exact functions and abilities of MGCs remained scarce and left many questions open. “Because they are so big and derive from multiple phagocytic cells, it had been suggested that they may act as specialized disposal units for particular forms of waste. But a definitive confirmation or a molecular and cellular basis for this theory was so far lacking,” explained Dr. Admar Verschoor, co-author of the study and group leader at the TUM’s Institute for Medical Microbiology, Immunology and Hygiene.
Systemic amyloidosis as a model system
To investigate the function of the giant cells, he and his team conducted a series of sophisticated cell culture experiments, while at the same time also looking into systemic amyloidosis, a disease in which extensive protein deposits gradually disrupt the function of affected organs, such as the liver or spleen. The team joined forces with Mark B. Pepys, a leading amyloidosis researcher from the University College London. In clinical trials, Pepys had already successfully tested a new treatment against the disease, which among other things promotes the formation of giant cells.
“The treatment´s exact mode of action remained still unclear in several regards. It turned out to be a perfect setting to apply our expertise in phagocytic cells and the immune system, and to more precisely examine how giant cells function,” remarked Ronny Milde, the study’s lead author and scientist at TUM. Using the combination of cell culture experiments and the amyloidosis disease model, the researchers were able to give the amyloidosis treatment a mechanistic basis, revealing how giant cells get a little help from something called the "complement system" in picking up the bulky and disruptive protein deposits.
Giant cells dispose of bulky waste
“Complement acts a bit like the butter on our bread; Just like bread tastes better with a bit of butter on it, the pathogenic protein deposits in amyloidosis become more attractive for giant cells with a bit of complement on them. Besides promoting the formation of giant cells, the new treatment also leads to complement deposition onto the amyloid deposits,” explained Admar Verschoor. The researchers observed under the microscope how the large phagocytic cells surround and destroy the disruptive protein clumps. “Our studies show that giant cells are particularly well suited to remove large targets that are complement-marked, explaining why they can act so effectively in the promising new treatment for systemic amyloidosis,” pointed out Verschoor.