May 23 2016
Researchers at A*STAR's Institute of Molecular and Cell Biology (IMCB) have developed advanced microscopy technologies to monitor embryo development in real time, revealing how mammalian cells differentiate during the earliest stages of embryonic life. These findings, coupled with the novel imaging technique, hold great potential in shaping how assisted reproduction procedures such as In-Vitro Fertilisation (IVF)[1] and Preimplantation Genetic Diagnosis (PGD)[2] are performed, and making these procedures more effective.
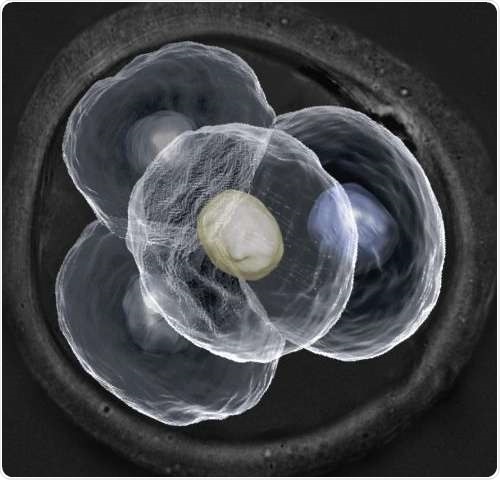
Microscopic image of a four-cell mouse embryo. Credit: Dr Nicolas Plachta
Across the globe, more women are relying on assisted reproduction procedures. Singapore alone, for example, has carried out over 6000 assisted reproduction cycles in 2015, an increase of more than 1000 cycles from 2012[3].
Current IVF procedures assess whether an embryo is suitable for implantation into the mother largely through observable measurements such as gauging if the rate of growth of the embryo is normal. PGD of embryos, on the other hand, is carried out by analysing a randomly extracted embryonic cell for genetic defects, with the assumption that all cells within a preimplantation embryo are identical and that the removal of a single embryonic cell would not affect the overall development of the embryo after implantation.
Contrary to the current conception that every cell within a preimplantation embryo is identical, the team of researchers at IMCB has demonstrated that the cells are in fact differentiated and may play very different roles in later development. By designing new cutting-edge real-time imaging techniques, the researchers were able to examine every cell within a preimplantation mouse embryo without perturbing its development. They observed differences in the way which certain proteins in each cell bind to their target genes. The scientists also observed that there were variances in cell behaviour at every stage of the embryo's development. As mouse embryos bear strong resemblance to human embryos at early stage development, the findings indicate that cells within a preimplantation human embryo are also not identical.
The study, therefore, refines our understanding of early stage embryonic development and highlights how assisted reproduction procedures such as IVF and PGD may be further enhanced to ensure successful fertilisation, smooth pregnancy and childbirth. Further development of the real-time imaging technique may eventually enable fertility specialists to study the microscopic properties of embryos and decide more precisely if an embryo is suitable for implantation, or screen an embryo for genetic abnormalities using imaging lasers instead of physical manipulation. This would enable better quality control of embryos implanted in mothers hence potentially increasing the chances of success for these procedures through more efficient control of embryo quality.
Dr Nicolas Plachta, Senior Principal Investigator of IMCB, said:
Most laboratories conduct studies on embryonic cells via invasive methods which do not keep the embryo alive. Our lab is the only one in the world imaging single cells in live mammalian embryos at the quantitative level, which allows us to observe every cell within an embryo at every stage of its development. Our findings as a result of this advanced technique have put forth a new paradigm of knowledge that would encourage more detailed microscopic analysis for future assisted reproduction procedures.
Dr Sadhana Nadarajah, Director of KKIVF Centre and Senior Consultant, Department of Reproductive Medicine, KK Women's and Children's Hospital, said:
This novel method of screening embryos is indeed exciting. If it can be successfully used on human embryos, without affecting its successive growth, it will improve the technique of embryo selection in IVF.
Prof Hong Wanjin, Executive Director of IMCB, said:
In many developed countries like Singapore, women are having children later in their lives, which has been linked to declined fertility. As such, assisted reproduction procedures needs to be constantly improved and made more reliable to help women successfully conceive and sustain a healthy birth rate. Nicolas and his dedicated team of researchers have therefore made a significant breakthrough that could benefit the society greatly.
The study was published in the top-tier scientific journal, Cell, and was also featured as the cover of the journal.