EKF Diagnostics, the global in-vitro diagnostics company, announces the release of a significant critical evaluation of the Quo-Test analyzer carried out by the European Reference Laboratory for Gylcohemoglobin. This new evaluation represents the culmination of several years’ cooperation between EKF Diagnostics and the European Reference Laboratory to produce a world class Point-of-Care-Testing (POCT) HbA1c analyzer.
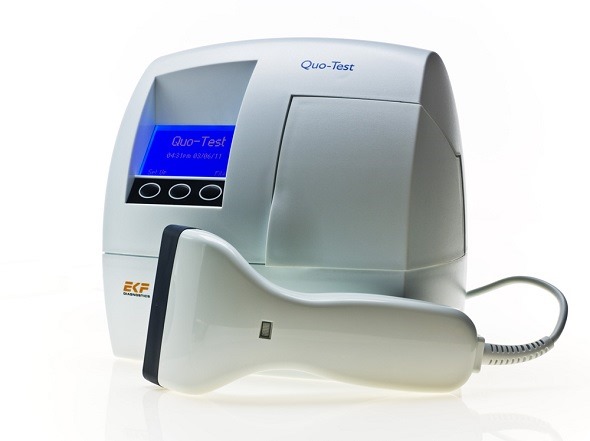
Image credit: EKF Diagnostics
Following CLSI (Clinical & Laboratory Standards Institute) guidelines and NGSP (National Glycohemoglobin Standardization Program) criteria for precision the Quo-Test HbA1c analyzer was found to have class-leading level of performance for precision with a CV (coefficient of variation) of 2.2% at 6.6% DCCT. According to the IFCC (International Federation of Clinical Chemistry and Laboratory Medicine) and the NGSP an HbA1c value of 6.5% DCCT indicates a patient can be considered to be diabetic.
This new evaluation also demonstrates that the unique boronate affinity fluorescence quenching technology employed by the Quo-Test helps to keep results consistent by minimising the effect of hemoglobin variants, showing very little difference compared to the market-leading HPLC lab systems. The Quo-Test showed no interference of the common Hb-variants HbAS, HbAC, HbAD, HbAE, HbAJ, elevated A2 (-thallasemia) and HbF <8.6%.
We are very happy with the results of this evaluation” said Global Diabetes Product Manager, Gavin Jones. “Following several disappointing evaluations produced by the European Reference Laboratory we decided to listen carefully to the advice they had to offer and incorporate that advice into our product development programme. What you are seeing in this evaluation is the result of that programme and we feel that we now have one of the most robust, accurate and precise POCT HbA1c analyzers on the market.”
The Quo-Test HbA1c analyzer has been designed for easy and reliable HbA1c measurement for the monitoring and management of diabetes in a point of care setting such as diabetes clinics and doctors’ surgeries.
The Quo-Test HbA1c is a fully automated hemoglobin A1c analyzer that uses patented boronate fluorescence quenching technology to measure glycated hemoglobin from a 4 μl sample taken from a finger prick or venous whole blood.
The full evaluation can be downloaded here.
For more information on the Quo-Test please visit http://hba1c-test.com/
About EKF Diagnostics
EKF Diagnostics Holdings plc, which includes the EKF Diagnostics, Stanbio Laboratory and DiaSpect brands, specializes in the development, production and worldwide distribution of point-of-care analyzers for use in the detection and management of diabetes, anemia and lactate. EKF products are sold in more than 110 countries around the globe, primarily through a network of specialist distributors.
Point-of-care: EKF Diagnostics designs and manufactures world-class diagnostic devices, as well as distributing rapid test kits for infectious diseases and pregnancy. The EKF analyzer range is used widely in GP surgeries, pharmacies, blood banks, sports clinics, hospitals and laboratories for glucose, lactate, hemoglobin, hematocrit and HbA1c measurement.
Central laboratory: EKF, through its wholly owned subsidiary, Stanbio Laboratory (Boerne, Texas, USA), manufactures a comprehensive range of clinical chemistry reagents, as well as associated analyzers. In addition, EKF Life Sciences (Elkhart, Indiana, USA) manufactures enzymes used in reagent development and also provides contract fermentation facilities.