This test, which we have branded SelectMDx for Prostate Cancer, came with the acquisition of a company in the Netherlands called NovioGendix. They developed a prototype assay, so they did most of the heavy lifting in terms of discovering the two specific biomarkers that are currently in the product. These biomarkers are tuned towards the detection of what I call clinically significant prostate cancer.
It is a very simple, straightforward molecular diagnostic test. Like most of the molecular diagnostic tests that are currently on the market, it is a PCR (polymerase chain reaction) based assay. After acquiring the company, we immediately started to commercialize this product and brand it as SelectMDx® for Prostate Cancer.
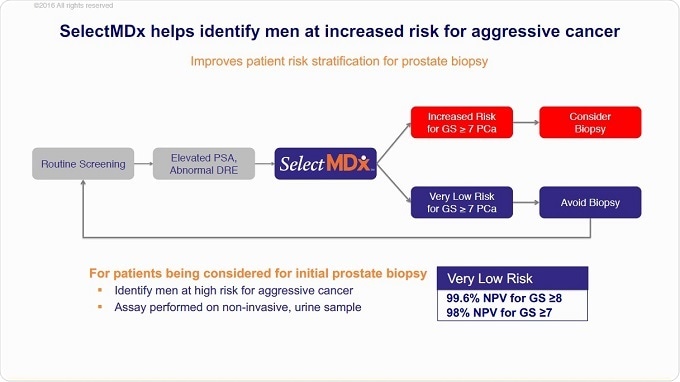
It works by detecting RNA in the urine of a man that has an elevated PSA level. A man is only eligible for the SelectMDx test, once the PSA level is elevated.
The standard of care is slightly different in the United States than it is in Europe. In the United States, they use the PSA (prostate specific antigen) test as a screening test. It is a very simple $15 blood test that detects circulation of the protein, PSA, in the bloodstream. If that’s elevated, which is defined as higher than 3mg/mL, it indicates that something may be wrong with the prostate. It wouldn’t necessarily mean the man has prostate cancer; it could be an infection of the prostate or prostatitis, for example.
Elevated PSA levels does not mean you have prostate cancer, an enlarged prostate, for example, can also result in an increased PSA level. In contrast to the United States where PSA screening is commonplace, in Europe, if a patient has clinical symptoms or is worried something might be wrong with his prostate, he will first visit his GP (General Practitioner) to discuss his symptoms. The GP could consider measuring the PSA and if it’s elevated, the patient might receive a referral to see the urologist. Based on increasing PSA levels combined with other clinical manifestation, the urologist might decide to biopsy the patient. The biopsy of a man’s prostate is the standard of care to confirm a prostate cancer diagnosis.

Today, we know that the vast majority of men with an elevated PSA level undergo a biopsy procedure, but only a small portion of them will be diagnosed with prostate cancer, based on the histopathology results. The biopsy procedure, which is a rectal procedure, involves 12-cores being taken by needle from the prostate, which are sent to a pathology lab for review.
The pathologist analyses the samples using a microscope and checks for the absence or presence of cancer cells. When cancer cells are present, it’s obvious, but if there are no cancer cells present, it may be that a needle has missed cancer, so to speak, or it could be that there is no prostate cancer in play whatsoever.
To put it into perspective, I'll give you some statistics. Each year, 1.3 million biopsies are carried out in the United States and based on the standard of care (the prostate biopsy procedure), approximately 220,000 men are diagnosed with prostate cancer. It's an invasive procedure and if only about 220,000 of the 1.3 million suspected cases do in fact have prostate cancer, that means that based on microscopic analysis, nearly 1.1 million men undergo a biopsy procedure when no cancer was present. By definition, that would not mean no cancer was present; however, it could be that they were just not able to detect it using the microscope.
Now, instead of patients with elevated PSA levels going straight to a biopsy procedure, we can provide our SelectMDx liquid biopsy test first. It’s a non-invasive test because it only entails collection of a urine sample, which we then test for the absence or presence of two prostate cancer-specific biomarkers in the urine.
When those biomarkers are present, you are most likely dealing with a high-grade disease − a clinical significant form of prostate cancer. If the biomarkers are absent, there is most likely no cancer or the patient is likely to have indolent prostate cancer.
It's an early stratification test, to identify the patients that most likely are dealing with aggressive prostate cancer.
How accurate is SelectMDx and how has it been validated?
The test had been validated in a multi-center trial of more than a thousand patients before we launched the product to the market. Patients with elevated PSA levels, with and without a history of prostate cancer were included in these studies.
The negative predictive value of the SelectMDx test today is 98%. By using this test, we can identify men that need to be followed up by a prostate biopsy procedure or MRI scan, because once a patient’s result is negative, the likelihood they have a high-grade disease or clinically significant cancer is very low.
Reducing unnecessary biopsies is the main purpose of the test. As a physician would tell you, as stated in the clinical guidelines, if the PSA level is elevated and/or another clinical manifestation, a patient is put on the list for a prostate biopsy, which is not risk-free procedure. Up to 45% of men who undergo biopsies experience rectal bleeding and up to 6% are hopsitalised for infections. We even see sepsis in about 3% of men hospitalized for infection.
Will the test be able to help select patients for MRI?
Absolutely, yes. MRI is considered to be the new tool for urologists. This may be my view on the world of the physician, but in general, physicians like to see pictures.
You can imagine if you’re carrying out a biopsy and they provide you with a picture of the tissue where it is possible to see whether cancer cells are present or not. Similarly, an MRI scan generates pictures where you might be able to see the cancer.
In some respects, an MRI scan is also quite often perceived as invasive for the patient as being placed in a tube can have a psychological impact.
Data with the SelectMDx test, which we recently present at the American Urological Association’s (AUA) annual meeting in Boston, we showed that the test could identify men who are most likely dealing with a high MRI score, which is expressed in the PI-RADS scores, divided into classifications one, two, three and four. If a patient is in category three and four, they most likely have clinically significant cancer.
The data we presented at the AUA, clearly shows a very strong correlation; if the SelectMDx test is positive, so to speak, the patient will most likely also be positive on the MRI scan.
A very important element here is that the focus today is on men with clinically significant cancer, because they will need treatments. With low-grade otherwise known as indolent cancer, there's no urgent need for intervention. Most men will develop prostate cancer in their lifetime, without experiencing any clinical complications, so there's no urgency for intervention in men with low-grade disease.

Has the cost effectiveness of SelectMDx been analyzed? How does it compare to PSA testing alone?
I mentioned the price of the PSA test, which is $15 or maybe a little bit more in the UK. The PSA test is still the gold standard for the urologist, so that's mainly the tool they use to identify men that might have an issue with their prostate. The next step is the biopsy procedure. That’s not only quite invasive, but also more expensive. The price of the biopsy varies between countries, but, for example in the United States, it is $3,000 or more.
In the UK, the SelectMDx test is £225. I don't know exactly how much a 12-core biopsy costs in the UK, but there’s a significant saving if you use the SelectMDx test first. We have published data to that effect in the British Journal of Urology International, where we demonstrated a significant saving of up to €125 per patient and an increase in quality of life if a SelectMDx test is used first, before proceeding to biopsy.
What do you think the future holds for prostate cancer diagnosis?
If you go back many years ago and look at the field of infectious disease, the main tool they used was culturing of bacteria or viruses. That whole world has changed now, because it has since been molecularised − molecular diagnostic tools are now used to identify patients with bacterial, fungal or viral infections.
As I mentioned before, if you look in oncology pathology today, 95% percent of the diagnosis is still based on the use of the microscope. Diagnosis of cancer is still very traditional: if there's a suspicious mass, they take a biopsy and then they send the tissue to the pathologist for review. At the end of the day a pathologist is always needed to confirm the cancer diagnosis, even when a molecular test is positive.
But as you can imagine, there's absolutely an unmet need for molecular diagnostic biomarker tests, to improve upon the patient diagnosis, prognosis or the monitoring of patients with cancer. That whole field is open and biomarker assays, DNA or RNA assays, will provide physicians with more actionable and accurate information for early intervention or treatment decisions.
In the world of prostate cancer today, you will see a high influx of new molecular diagnostic tools coming to the market to support physicians in managing their patients.
What is MDxHealth’s vision for the future?
As a company, we focus on uro-oncology and we absolutely believe this is a wide-open space for us to develop new tools for the diagnosis of patients with kidney cancer, bladder cancer and prostate cancer, but more importantly, as you have probably noticed, more and more expensive treatments will come to market for the treatment of cancer.
Most of those treatments involve specific drugs that require specific biomarker assays to upfront identify patients that most likely benefit from that particular treatment or assays to monitor the patients over time to see what the response is to that particular drug.
We will also see many new cancer-specific biomarkers assays coming to the market to guide physicians who most likely will benefit from a certain drug. As many of the new orphan drugs are very expensive and not all patients will respond to these drugs. These type of predictive assays will result in better treatment and cost savings for the healthcare providers.
Where can readers find more information?
About Jan Groen, Ph.D.
President and Chief Executive Officer, Executive Member of the Board

Dr. Jan Groen joined MDxHealth in 2010 and has more than 30 years of experience in the clinical diagnostics industry, with a particular focus on emerging technologies, product development and commercialization.
Dr. Groen was previously the president of Agendia, Inc. and COO of Agendia B.V., responsible for their United States and European diagnostic operations, respectively. Prior to this, he served as VP of Research & Development at Focus Diagnostics, Inc., a subsidiary of Quest Diagnostics, in California.
Dr. Groen has held numerous management and scientific positions at ViroClinics B.V., the Erasmus Medical Center, and Akzo-Nobel. Dr. Jan Groen is a supervisory board member of IBL International B.V.
Dr. Groen holds a Ph.D. degree in Medical Microbiology from the Erasmus University Rotterdam, a BSc in Clinical Laboratory Studies and has published more than 125 papers in international scientific journals in the field of clinical diagnostics.