An interview with Prof. Igor Sokolov, Tufts University conducted by April Cashin-Garbutt, MA (Cantab).
Can you please explain how you use AFM to study the nanomechanical properties of cells related to aging processes and cancer?
Force microscopy is a technique which would probably be best described with the help of a small finger with an apex just a few atoms in size that can touch objects. This is a learning finger.
It is exactly like when we learn things about the world around us using our fingers. You can touch, push and scratch; you can see how much your finger sticks to objects. Biological cells are an example of these objects. We study physical characterisics of cells using this learning finger, the AFM probe. We look at aging and cancer because these are probably the most interesting and challenging topics.
The Physics of Cells in Cancer and Aging
The Physics of Cells in Cancer and Aging from AZoNetwork on Vimeo.
Defeating aging is something beyond our imagination because, even if you think about some of the craziest science fiction, there is literally no description of a future for humans with immortality, because, people do not know how to handle this.
For example, it is an open question whether aging is pre-programmed. We do not know that. There is a lot of biochemical theories defining what aging is. Biology is essentially biochemistry. These days we can study physics of objects, particularly on a small scale such as the cellular level cells. To do that, you need something like a finger, AFM probe because it provides a physical touch, a must to study physical information.
As you do that, you learn first the mechanics of cells − how responsive they are to external loads, pressure, scratching, and even tickling. As almost a joke, we came across an interesting phenomenon. When we started to poke cancer and normal cells (human cervical epithelial cells) with a sharp AFM probe, the cancer cells started to crawl away, whereas normal cells remain still.
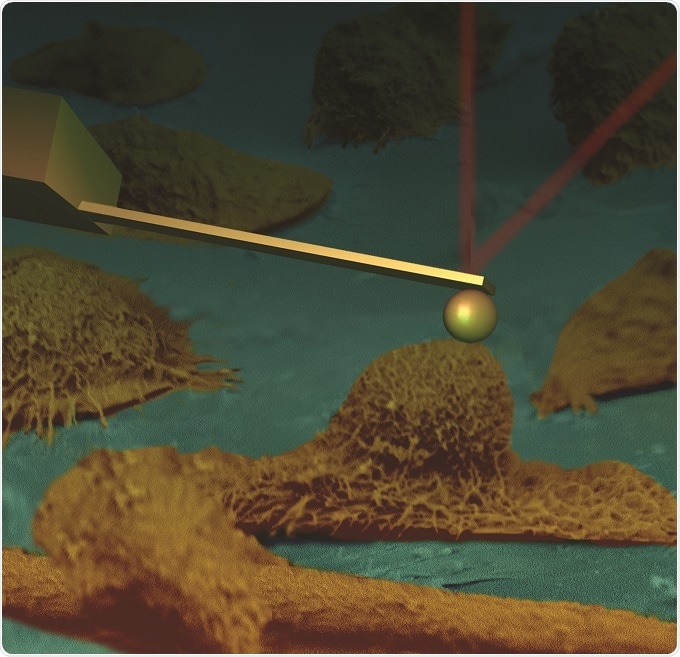
A collage of an AFM cantilever touching human cervical epithelial cells imaged with scanning electron microscope (SEM).
If you use a dull probe or sphere, both cell types were okay. It is almost like the cancer cells don't like tickling or something like that. This is an observation we have not published. We still do not know why they behaved like that. This is just an example of how many things we don't know about the physics of cancer.
It is also not known what cancer is, whether it is a switch or a mutation, as generally believed. From a physical point of view, it is literally unknown and I think force microscopy is the only technique capable of enabling the comprehensive study of cells, including the mechanics of the cell body and physical properties of layers surrounding cells.
What new modes have you developed to use AFM to study mechanical properties of both living cells and materials/polymers?
One mode is called FT-nanoDMA. FT stands for the Fourier Transform. There are different ways you can measure cell mechanics, but one of them is very natural; you push a cell and start to vibrate your probe a little bit with different frequencies, and then you measure the response.
Usually, it is done sequentially. You vibrate at one frequency, then at another frequency, and another and so on. It takes time. But cells are alive, it is not very measurement-friendly because it keeps changing.
What we did was simply send all these vibration frequencies altogether at once. It resulted in rather fast measurements. The area of contact between the AFM probe and sample stays almost the same, which is paramount for qantititative measurements. Our estimations show that the speed of measurements has increased almost two orders of magnitude.
It is a similar improvement with the spatial resolution; we found almost a hundred times increase inresolution. A hundred times higher resolution is a fairly big difference; it is the difference between optical and electron microscopy, for example.
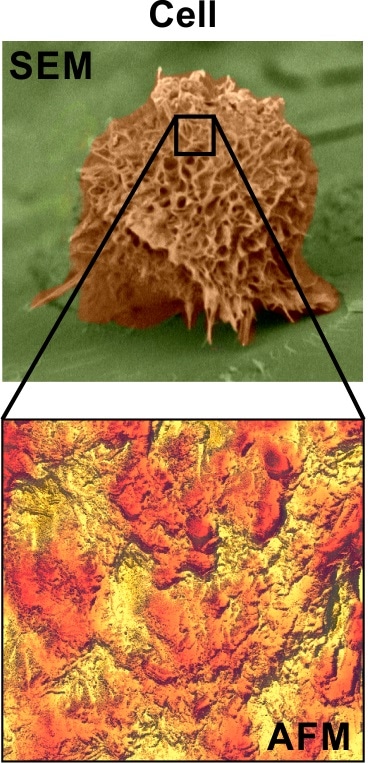
Comparison of SEM and AFM images of cells. The AFM image (bottom) is a zoomed area of the cell imaged with SEM (top image).
We published this just over a year ago. Practical implementation requires some additional hardware to exisiting AFMs. Right now it is commercialized by NanoScience Solutions, Inc.
Now, just literally two days ago, I received notice of acceptance of our other paper describing another AFM mode. When you disconnect an AFM probe (your learning finger) from a sample surface, you usually pull up some molecules and a little bit of surface itself. All that information is there.
However, previously, that information was filtered out because it was treated as noise in the existing sub-resonant tapping modes. What we did was process that information before it was filtered out. It required to attach some new faster electronics to the existing AFM. So it has been done on Bruker AFMs, but it should work on all AFM, even old ones. The results are much better than we expected.
We can record up to eight new channels of information, much faster and with less artefacts compared to the existing sub-resonant tapping modes. This mode has been also already commercialized by NanoScience Solutions, Inc.
This is a really new and promising mode. We are currently applying this for cancer detection, together with medical collaborators.
What were the main challenges you had to overcome?
We have had to face both technical and societal/psychological challenges. Regarding the technical challenges, atomic force microscopy is a fairly young technique. Although it is almost 30 years old, it has passed through the same stages that all new techniques.
In the beginning, there was a lot of excitement, abuse of the technique, and bursting of this bubble of initial interest. Now it has slowly started to become generally accepted method. Still, it requires a lot of learning, and that is where students have to be prepared. Getting just a picture with AFM is not a big deal; it simply records force interaction between an AFM probe and sample surface.
However, if you don't know which force it could be, then you may get some artefacts and incorrect results. That is the biggest difficulty. AFM it is not just a push-button technique. You have to interpret the obtained image. Of course, sometimes there are some simple cases, but if you are really at the frontiers with this technique, that's the difficulty.
The second difficulty relates to research and education. It is interesting how the education and research are going together. Both keep changing. It was different literally five or ten years ago. In the United States, if you are in a tenure-track position, you have to fight for money. You have to write grant applications and papers. It is very hard to find time to learn something deep and new, to go to the lab to do it yourself.
Therefore, you typically rely heavily on students. But they have their own agenda, which is to get a PhD. If they see a new or more complicated approach compared to what traditionally published, they try to get around it to get faster results. If the professor insists, they may say "I tried and it did not work well", and then they use the simpler approach while professors simply don't have time to do that themselves.
That is why the popular approach is the simplest one. This is the problem of AFM because it captures so much complexity - you literally get direct information from interactions, even atomic interactions in the nanoscale. You get gigabytes of data. If you really want to process all those data it requires a lot of knowledge and interpretation of what is being observed.This is what I think is slowing down general acceptance of this technique.
How can this be overcome?
I think it is simply psychological. Some people will realize that to get something good with this technique you need to invest a little bit more time. On the other hand, the technology keeps developing and becomes more user-friendly.
Right now, I can compare several new AFMs by Bruker, for example, with automatic car transmission. You do not need to have the knowledge of driving a standard car. Many people like it. It feels faster to learn. Personally, I like some tweaks, but at the same time, I hate driving a non-automatic car.
I think that with time, people will definitely get a truly user-friendly AFM. However, the physics and need in knowledge of forces at the nanoscale are still there, and will always be there. You need to really learn and understand what is measured and that is still a difficulty.
Can you please outline how you are studying viscoelastic properties using the nanoDMA?
You simply push a surface with a predefine force to develop some area of contact, and then, you oscillate the probe with several frequencies altogether. Right now, we work with ten frequencies, which is sufficient for many applications.
We can do more. It is still about balancing the cost of the hardware and how many frequencies you need analyze at the same time. This frequency-based method is the most model-independent one to characterize materials, particularly soft materials like cells.
The range of frequencies is currently from single Hz up ~500 Hz. The maximum frequency is defined by the previous study of polymers. For polymers, there is a large database of information of their viscoelastic properties up to 300 Hz, which has the gold standard. There is some indication that it might be interesting to go to very high frequencies. There are other people doing research in high frequency viscoelastic measurements, but I think low frequency is important, in particular for biology. It is important for cells, for example, because they are soft and they don’t like being shaken at mega-Hertz frequencies.
How has AFM directly advanced or helped your research?
AFM can measure not only mechanics and physical interactions, but it can measure electrical properties, tribology, and measure how durable the surface is, etc.
AFM, which is the same as scanning probe microscopy, is a family of different techniques. Although we use quite a lot of techniques, I don't know of any other technique capable of getting such a large amount of various information about surfaces. I would say it is my favorite technique, although we are using many different ones to study the self-assembly of molecules on the surface and the properties of cells and tissues.
I recently started to study organoids, a completely different approach that is very popular these days. This is because, when you look at a tumor, for example, it is not even clear which cells are cancer cells and, which are not. This became almost impossible to identify at the single cell level. If you look at cells in a Petri dish (in vitro), they are separated in 2 dimensions on solid substrate. It may have a very little relation to real organs.
Nowadays what people do, they have started to build a kind of embryo/seed of organs using well-defined cells. This seed is called call organoid. All of the cells are known. The genetic make-up is known. You know which cells are normal and which ones are cancerous. This is a bridge between the two worlds of in vivo and in vitro.
What is the importance of meetings, like the AFM BioMed Conference, to you and the AFM research community?
It is very important because professors are busy, and with all the papers to read, it is very hard to get a good perception of the frontiers and what is going on in the community.
People do not publish negative results, but at conferences, you can at least mention it. From the learning point of view, negative results may be more educational than positive. Also, you can ask questions directly, which is very important.
Finally, such meetings are the place where you can convince people that it is worth looking at a particular approach. Maybe it is difficult, but it is worth it. Since such meetings involve professors and students, the professors have an additional motivation to push students to investigate more deeply. Of course, personal relations are important to us all, it also fuels collaborations.
Online meetings are fine for ongoing collaborations, but if you are thinking about something new, you need personal meetings. Furthermore, it is very important for students. Sometimes, students are very close with the collaborators such as their professors and their peers, but when they start seeing the world it typically motivats them a lot. Thus, such meetings are very important for them.
Conferences are very important in general, but particularly for force microscopy in biomedical applications. The biomedical arena has been number one on the horizon of force microscopy right after it was created. Yet therewere so many difficulties that up to now there is literally no medical application of atomic force microscopy.
I think it is extremely important to orginize such conferences because we are trying to help to give birth to this area of medical applications. I am trying to work with doctors and I see how difficult it is. It is not only about different vocabularies, it is a different universe, different paradigm and different goals..
To effectively communicate the great benefits of using AFM in medical area, we need to define a topic which may be particularly interesting for medicine. This type of conferences is a great place to discuss these topics, and may be to endorse it by the AFM community.
What direction do you see, or would like to see, AFM going in the next five years? What do you see as the next big thing for AFM?
There will probably be faster electronics, more sophisticated algorithms, and more user-friendly interfaces. Increased speed is one of the most interesting parts of the discussion − whether force microscopy will be as fast as real-time video? I think it will, but it would have limited applications , not for all samples.
Secondly, control of the AFM probe is also very important. The probe that touches the surface is typically controlled through a feedback. For example, the load force acting between the probe and surface is kept constant during scanning by a feedback system.. At present, control feedback system is rather basic compared to what is used in the communities dealing with feedback controls.
It is definitely time for the control system to be more sophisticated. As a result, the speed of force microscopy would increase enormously, and at the same time, it will preserve the sample. I think there will be new controls implemented in future AFMs.
Where can readers find more information?
About Prof. Igor Sokolov
 Igor Sokolov received his B.S. in Physics from St. Petersburg State University, Russia in 1984, and earned his Ph.D. from D.I. Mendeleev Central Institute for Metrology the Soviet Bureau of Standards (Russian NIST), Russia in 1991. In 1992, he was the recipient of the E.L. Ginzton International Fellowship Award from Stanford University for his work on atomic force microscopy.
Igor Sokolov received his B.S. in Physics from St. Petersburg State University, Russia in 1984, and earned his Ph.D. from D.I. Mendeleev Central Institute for Metrology the Soviet Bureau of Standards (Russian NIST), Russia in 1991. In 1992, he was the recipient of the E.L. Ginzton International Fellowship Award from Stanford University for his work on atomic force microscopy.
Igor worked as a research associate in the University of Toronto's Physics and Chemistry Departments before moving to Clarkson University in 2000 to join their Physics Department, where he achieved the title of full professor and served as director of the Nanoengineering and Biotechnology Laboratories Center. Now he is Professor at Tufts University and Bernard M. Gordon Senior Faculty Fellow. During his career, he has consulted for many large corporations such as Proctor and Gamble, General Electric, Arkema Group, Inc. and Purdue Pharma.
He has 150+ refereed publications, including such journals as Nature, Nature Nanotechnology, Nature Methods, Advanced Materials, etc.. He holds 20 patents (issued and pending). Igor's current research focuses on nanomechanics of soft material, molecules and cells; atomic force microscopy; nanophotonics, and the studies towards understanding of nature of cancer, early detection of cancer based on altered biophysical properties; self-assembly.
About Bruker Nano Surfaces and Metrology

Bruker’s suite of fluorescence microscopy systems provides a full range of solutions for life science researchers. Their multiphoton imaging systems provide the imaging depth, speed and resolution required for intravital imaging applications, and their confocal systems enable cell biologists to study function and structure using live-cell imaging at speeds and durations previously not possible. Bruker’s super-resolution microscopes are setting new standards with quantitative single molecule localization that allows for the direct investigation of the molecular positions and distribution of proteins within the cellular environment. And their Luxendo light-sheet microscopes, are revolutionizing long-term studies in developmental biology and investigation of dynamic processes in cell culture and small animal models.