Feb 12 2018
Synthesis of a water-soluble warped nanographene and its application for photo-induced cell death
Graphene and its nano-sized little sibling, nanographene, are well known for their remarkable photoelectronic properties. However, biomedical applications are hampered by the insolubility of the materials, especially in water. A Japanese team of scientists has now introduced substituted “warped nanographene,” which is soluble in a broad range of solvents while maintaining its photophysical properties. In their publication inAngewandte Chemie, the authors also emphasize its photodynamic potential to selectively kill cells upon irradiation.
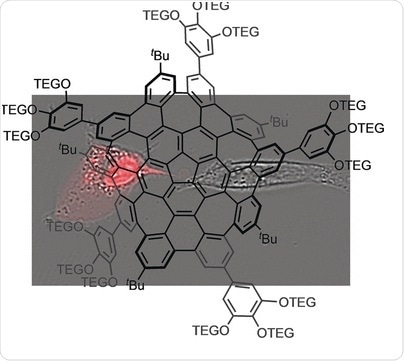
© Wiley-VCH
Nanographene has the hexagonal carbon lattice of graphene but consists of only a few carbon rings with tunable electronic properties. One of its big issues hampering widespread application in optoelectronic devices or biomedicine is its insolubility. Therefore, to suppress stacking and aggregation, a new type of nanographene with a bended structure has been synthesized, the so-called warped nanographene. Kenichiro Itami at Nagoya University, Japan, and his colleagues have now found a way to furnish the warped nanographene even further to obtain a fully soluble, amphiphilic product. The new structure was biocompatible, but upon irradiation it killed its host cell. This effective photosensitization behavior could inspire future research in photodynamic cancer therapy, the authors believe.
The poor solubility of graphene-like materials has been regarded problematic since the discovery of graphene as an intriguing one-layer carbon modification in 2004. To improve solubility, Itami and his colleagues have developed warped nanographene molecules with chemical substituents at the outer rim of the aromatic structure. The substituents were introduced by the relatively simple and powerful strategy of borylation. Once the molecule is borylated, the boron substituent can be replaced by other substituents, in this case, by an aromatic molecule bearing highly soluble tetra(ethylene glycol) chains (TEG). Applying this substitution–replacement strategy twice, the scientists accomplished the synthesis of a warped, i.e., bended, nanographene molecule that was stable in a broad range of solvents including water. Excited with a laser, it exhibited green fluorescence.
This fluorescence points to applications in biology, for example, as a dye in bioimaging. A further application came rather unexpected, the scientists reported. Upon excitation, the molecule, which was otherwise not harmful to the cells, killed the cell population of the human HeLa cell line to almost 100 percent. The authors proposed:
Although the mechanism is unclear, the relatively high efficiency of the singlet oxygen generation of [the soluble warped nanographene] may contribute to its HeLa cell death.
Thus, a mechanism similar to dye sensitization and production of reactive oxygen species can be assumed.
These second-generation nanographenes combine the remarkable optoelectronic properties of graphene with biocompatibility. They may well play a future role in bioimaging, photodynamic therapy, and similar applications.