Scientists have now bred bacteria that can create microscopic high energy carbon rings. This scientific breakthrough comes from Caltech scientists who have tweaked the enzymes of these bacteria in ways so that they can provide new molecular structures. The study titled, “Enzymatic Construction of Highly Strained Carbocycles,” was published in the latest issue of the journal Science.
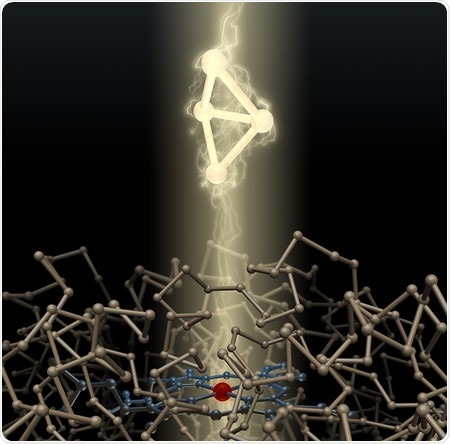
An artist's depiction of a bacterial enzyme and the high-energy carbon ring it created. Credit: Caltech
The team of scientists, by engineering the enzymes of the bacteria has now created a strain of bacteria that can make for themselves small energy-packed carbon rings. These carbon rings can be used as bases for making other chemicals and materials explain the scientists. These rings are difficult to make in the labs they said.
Linus Pauling Professor of Chemical Engineering, Bioengineering and Biochemistry worked at the lab of Frances Arnold at Caltech using techniques previously used by Arnold’s lab to create bacteria with properties they desire. Earlier techniques have altered bacteria to create strains that produce carbon-silicon and carbon-boron bonds. These bonds are not found naturally.
Now the team has made these bacteria make carbon rings which are also rarely found naturally. Arnold explains that these bacteria are now able to “churn” out the “versatile, energy-rich organic structures” that “chemists struggle to make.”
The scientists write in their paper that they engineered the enzymes within bacteria - Escherichia coli to create bicyclobutanes. These molecules contain four carbon atoms arranged so they form two triangles that share a side. This is much like a square with a semi-fold across a diagonal. These bent angles of the carbon atoms put them in great strain and thus the molecules are difficult to make. They are also rarer in nature unlike other carbon rings such as cyclohexanes and cyclopentanes. A large amount of energy is required to bend the carbon atoms at the folds. Once created these bent angles act as sources of energy like a wound up spring, explain the scientists. These bent angles can also transmit electricity. These bicyclobutanes can now be used in several chemical products such as pharmaceuticals, agrochemicals, and other smart materials that can respond to the environment.
Kai Chen, lead author on the paper and graduate student working at Arnold’s lab, said that the enzymes could be engineered successfully so that they can make “crazy carbon rings under ambient conditions”. He added, “This is the first time anyone has introduced a non-native pathway for bacteria to forge these high-energy structures.”
The bacteria E. coli was given a gene that codes for cytochrome P450 enzyme. They could create the tiny rings with exact precision in its exact chiral form (molecules are either right handed or left handed according to their chiral forms).
Chiral forms are important in pharmaceutical drug chemistries. According to the researchers, this study is a breakthrough because in future the bacteria could be harnessed and programmed to create the exact molecules required.
The study was funded by the National Institutes of Health, the National Science Foundation, and the Donna and Benjamin M. Rosen Bioengineering Center.