A study conducted at the University of Illinois, Chicago has explained for the first time why the CRISPR gene editing tool sometimes fails to work. The study authors also suggest steps that can be taken to stop the tool from failing, which happens approximately 15% of the time.
 Image Credit: Meletios Verras / Shutterstock
Image Credit: Meletios Verras / Shutterstock
CRISPR gene editing is used by researchers to remove undesired genes or genetic material from DNA and sometimes to insert the genes or sequences they do desire.
The unwanted DNA is removed by an enzyme called Cas9, which cuts the unwanted material on either side. The cell then either repairs the DNA strand by joining the two cut ends back together or the cell dies.
As reported in the journal Molecular Cell, senior author Bradley Merrill and colleagues showed that when the gene editing tool fails, it is often because Cas9 persistently binds to the site where DNA is cut, which prevents the enzymes involved in DNA repair from reaching the cut site.
Merrill says that, until now researchers, have not understood why this gene editing process sometimes randomly fails.
The study also showed that the bound Cas9 cannot proceed to making any further cuts in the DNA strand, thereby limiting the tool’s efficiency.
In addition, Merrill and colleagues found that Cas9 was likely to be ineffective at DNA sites where RNA polymerases were inactive.
The researchers then showed that helping Cas9 anneal to just one of the DNA strands of the double helix led to Cas9−RNA polymerase interaction, which helped Cas9 become effective at gene editing.
I was shocked that simply choosing one DNA strand over the other had such a powerful effect on genome editing. Uncovering the mechanism behind this phenomenon helps us better understand how Cas9 interactions with the genome can cause some editing attempts to fail and that, when designing a genome editing experiment, we can use that understanding to our benefit."
Ryan Clarke, Lead Author
Clarke adds that the new understanding should help those working in genome editing to make the process more efficient and safer to use in future clinical studies.
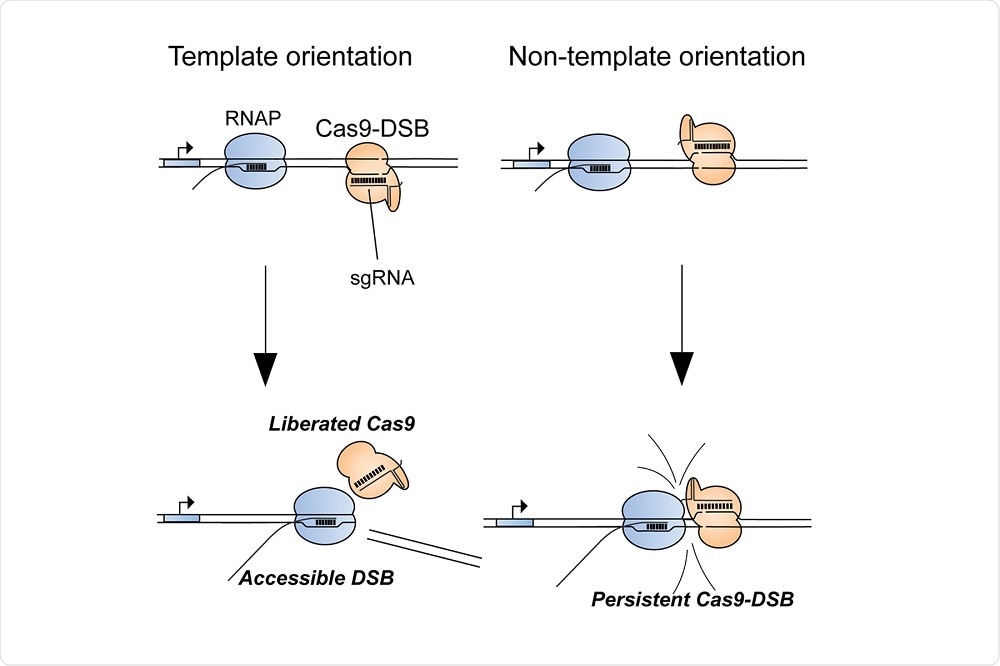 Image Credit: Ryan Clarke, et al
Image Credit: Ryan Clarke, et al