The tumor microenvironment is a general term describing all the things that are a part of the tumor but are not cancer cells. This includes healthy epithelial cells, cells of the immune system, blood vessels and the extracellular matrix (ECM); a scaffold of proteins that surrounds every tissue in the body, including tumors. The ECM also includes non-cellular features, such as the percentage of oxygen.
 Image Credit: Giovanni Cancemi / Shutterstock
Image Credit: Giovanni Cancemi / Shutterstock
The tumor microenvironment is a hypoxic region containing a strong signaling group of cells that can withstand the mechanical forces and chemical signals surrounding the tumor.
This environment is important because it determines how the cancer cells will respond to treatment and thus, the prognosis of the disease. Patient outcomes are not just based on the size of the tumor and the number of mutations it contains. Instead, outcomes have a lot to do with the dialogue between cancer cells and the rest of the body, especially the cells and proteins found within the tumor microenvironment.
A central goal of my laboratory is to distinguish between promotion and resistance. We do this by building experimental systems in the laboratory wherein we can determine whether a given factor in the microenvironment is important or not in the growth of the tumor.
If a particular factor is found to be important for tumor growth, we might want to inhibit it. Equally, if a factor is inhibiting the growth of the tumor, we might want to strengthen its activity to prevent metastasis.
What is currently known about the breast cancer microenvironment and the stages of tissue invasion?
Breast cancer has two distinct forms; in situ and invasive. Ductal carcinoma in situ (DCIS), for example, is a fancy phrase for breast cancer cells that are surrounded by some of their normal tissue constraints. This includes myoepithelial cells and the basement membrane; a specialized set of proteins that surround the ducts of the breast.
As long as the cancer cells are inside both the myoepithelium and the basement membrane, the pathologist will diagnose it as ductal carcinoma in situ. Over 99% of these patients will have great outcomes.
Where it becomes challenging is when the cells have broken through the myoepithelium, which is clinically defined as invasive breast cancer. Whether or not the cancer cells have broken through the myoepithelium is the deciding feature between whether a diagnosis of DCIS or invasive breast cancer is made.
Regarding the breast cancer microenvironment, our lab and many others have proven on numerous occasions that the proteins surrounding the tumor are very important.
For example, collagen-1 is a very abundant protein in the human body. When a woman detects a lump in her breast, she's feeling an abundance of collagen. This abundance is not like a couple of extra molecules – it is so much extra protein that it causes a lump. Work from labs such as Patricia Keely’s have shown that this collagen-1 is a very strong promoter of cancer invasion.
Our research has considered this as well. We recently showed that if you put cancer cells near collagen-1, they will align the collagen-1 into something resembling railroad tracks that they then use to migrate out of the tumor.
The myoepithelium, on the other hand, is resisting the invasion of the tumor. Therefore, if there is a myoepithelium, the cancer is not invasive, whereas if there is a break in the myoepithelium, the cancer cells migrate out into other tissues. We focused this project on trying to understand how that worked, how to think about it, and what we could do next.
 Image Credit: Giovanni Cancemi / Shutterstock
Image Credit: Giovanni Cancemi / Shutterstock
Please describe your recent research surrounding breast cancer.
We knew that the presence and integrity of the myoepithelial layer was the difference between a benign disease and an invasive one, but we wanted to understand how it worked.
We started by building 3D culture assays, which would allow us to grow whole tissues in the laboratory. You can think of tissue organization as being like raisins in a Jell-O mold; you have these structures growing within a three-dimensional environment of proteins.
We used various genetic tricks to visualize the cells that were trying to invade in one color and the myoepithelial cells in another color.
What was immediately striking was that the myoepithelium was actively responding to the invasive behavior of the cancer cells.
We would see the invasive cancer cells marching forward, and the myoepithelium reach out, recover them, and push them back inside. Most of the time, the myoepithelium would win.
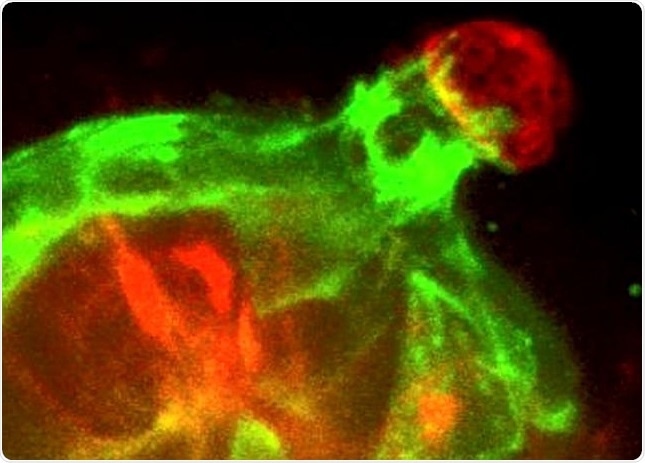
Real-time 3D confocal time-lapse movie of Twist1-expressing epithelial cells (red) invading into the surrounding extracellular matrix and then being restrained and pulled back by normal myoepithelial cells (green). Image Credit: Katarina Sirka.
Having seen this, we were really excited, but first, we needed to test whether this was a correlation or something that was causally related. We arranged different ratios of myoepithelial cells to invasive cells and were able to show that the more myoepithelial cells you had, the less invasion that occurred up to the point where you had a complete myoepithelial layer with essentially no invasion.
The next step was to understand the molecular basis of this interaction. We inhibited different pathways involved with either the adhesion between the myoepithelial cells or the contraction of the myoepithelial cells. Our results showed that both of those features, adhesion, and contraction, were required for the myoepithelium to act as a barrier to invasive behavior.
Scientists Discover a Dynamic Cellular Defense Against Breast Cancer Invasion
How do your findings differ to previous research, and what does this mean for patients?
How is it different? The analogy we like to use here is that people knew that the presence of the myoepithelium correlated with patient outcomes and that it was sufficient for a diagnosis of invasive breast cancer, but what they didn't know was how those myoepithelial cells worked.
Before our research, it was possible this was purely a correlation. Ours is one of a very small number of papers that have tested whether the myoepithelial cells are functionally involved in resisting invasion, or whether they were just a good marker for the presence of invasion.
The most important concept that this paper introduces is the myoepithelium as a dynamic barrier. The analogy I like to use is between that of a castle wall. Imagine the outer wall in a castle; if that wall is breached, the invading army can simply run through it. There's no further resistance from a wall after there's a big hole in it.
However, what we showed is that the myoepithelium is better thought of as a soccer or football defense. The cancer cell can break though partially, but the myoepithelial cells will actively migrate, try to push it back inside.
The idea of a dynamic response to changes within a tissue is having an impact on research into other diseases. Scientists are now starting to consider the involvement of cells and epithelial barriers in a completely different way.
In answer to your second question, the key concept here is that we've increased our understanding of how the myoepithelium works and are now actively collaborating with pathologists to test whether we can leverage this understanding of how the myoepithelium works to provide a more individualized and personalized assessment of the risk of recurrence for women with breast cancer or DCIS.
What are the next steps for your research?
My laboratory studies metastasis; that’s the number one thing we're interested in studying. We're trying to understand how normal cells, through a series of mutations, acquire the ability to spread through the body, survive an unfamiliar environment, invade the immune system, and eventually grow tumors in distant organs.
We're primarily focused on this question concerning breast cancer because it is in this disease that we think we could make the biggest and most immediate impact on patient outcomes with more research.
At the moment, we are trying to understand how the cells of the immune system interact with cancer cells as they're spreading through the body.
We're also working to understand the way in which molecular pathways in the cancer cell allow it to survive as it spreads through the body and transitions into a distant organ.
If we can understand all of this, and model the data using computational systems, we should be able to have a real impact on patient outcomes.
Where can readers find more information?
About Dr. Andrew Ewald
 Dr. Ewald received his undergraduate degree in physics with honors from Haverford College. He earned his Ph.D. in biochemistry and molecular physics from the California Institute of Technology.
Dr. Ewald received his undergraduate degree in physics with honors from Haverford College. He earned his Ph.D. in biochemistry and molecular physics from the California Institute of Technology.
He completed postdoctoral work with Zena Werb in mammary biology and cancer at the University of California, San Francisco. Dr. Ewald joined the Johns Hopkins faculty in 2008.
He is a member of the American Association for Cancer Research, Society for Developmental Biology, and the American Society for Cell Biology.
Dr. Ewald's work was recognized with the 2011 Morphological Sciences Award from the American Association of Anatomists for his contributions to the field of epithelial morphogenesis.