Dec 3 2018
Multiple components of the nuclear pore complex and nuclear import machinery enable a protein called human myxovirus resistance 2 (MX2) to inhibit HIV-1 infection, according to a study published November 29 in the open-access journal PLOS Pathogens by Michael Malim of King’s College London, and colleagues.
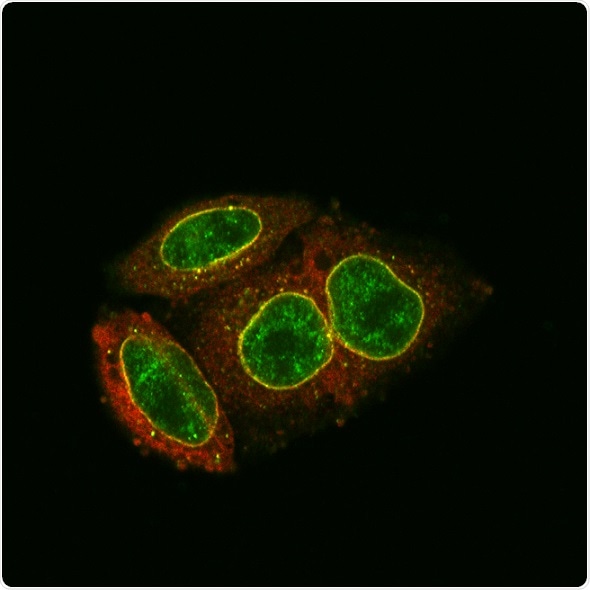
Antibody staining and light microscopy demonstrate the accumulation and co-localization (yellow) of MX2 (red) and NUP214 (green) at the nuclear envelope. Image Credit: Gilberto Betancor et al. (2018)
In eukaryotic cells, a membrane barrier called the nuclear envelope separates the nucleus from the cytoplasm. The movement of large molecules through the nuclear envelope and into the cell nucleus is regulated by large protein structures called nuclear pore complexes. To infect cells productively, HIV-1 must traverse the nuclear envelope to enable integration of the viral DNA into the genomic DNA of host cells. MX2, which is localized at the cytoplasmic face of the nuclear envelope, inhibits infection by blocking the nuclear import of HIV-1 DNA and preventing its accumulation within the nucleus. However, the precise mechanism of viral inhibition has not been clear.
In the new study, Malim and colleagues show that MX2 interacts with multiple protein components of the nuclear pore complex, as well as the nuclear transport receptor transportin-1 – a component of the nuclear import pathway. The findings suggest that TNPO1 and nucleoporins (particularly NUP214) help position MX2 at the nuclear envelope to promote MX2-mediated restriction of HIV-1. According to the authors, these new insights could lead to the development of more effective therapies for HIV-infected patients.