Two scientists at the Johns Hopkins University School of Medicine have unraveled aspects of how DNA organizes and preserves genetic information. Newly published research by Cynthia Wolberger, Ph.D., and James Berger, Ph.D., whose labs sit side by side, takes a closer look at how the puzzle pieces of DNA machinery fit together.
Credit: Evan Worden
New Study Uncovers Unexpected Change in DNA Organization
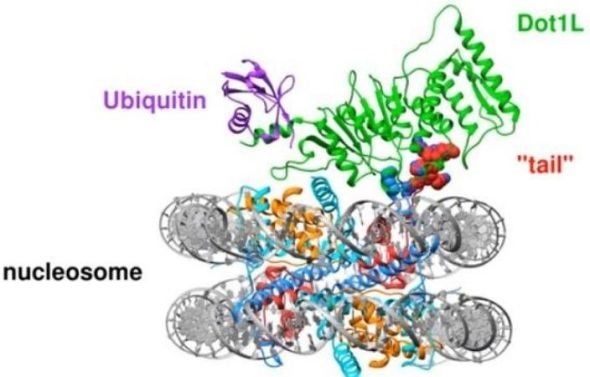
To turn genes in DNA “on” and “off,” enzymes in cells must interact with nucleosomes, which are complexes containing proteins that allow cells to organize their DNA. One such enzyme, Dot1L, is mutated in mixed lineage leukemia, a form of childhood leukemia.
A small protein tag called ubiquitin must first be attached to nucleosomes in order to help recruit Dot1L. However, how the Dot1L enzyme physically connects with the nucleosome or with the ubiquitin tag was not clear until Cynthia Wolberger, Ph.D., professor of biophysics and biophysical chemistry at the Johns Hopkins University School of Medicine, and Evan Worden, Ph.D., a postdoctoral fellow in her lab, used an imaging tool called cryogenic electron microscopy (cryo-EM) to freeze molecules in a nucleosome and Dot1L to see how the two interact.
What they found in the study, which was published in February in Cell, was unexpected: Dot1L changes the shape of the nucleosome to bind more closely with the Dot1L enzyme.
The high-resolution images taken with cryo-EM revealed a dramatic never-before-seen change in the core of the nucleosome. As Dot1L connected, a tail from the center of the nucleosome swung up to secure the enzyme to its surface, causing a cascade of other changes in the nucleosome’s structure.
New Study Uncovers Unexpected Change in DNA Organization
Animation of the nucleosome ‘tail’ securing Dot1L after being recruited to the protein complex by ubiquitin. The movement of the tail is a never-before-seen change in the nucleosome’s molecular shape. Credit: Evan Worden
This observation, say the researchers, represents a paradigm shift in how we think about genetic illness, as changes in the nucleosome structure impact how our cells access their DNA. “It opens new doors for discovery we didn’t even know were there,” says Worden.
Specifically for childhood leukemia, the discovery of how the nucleosome changes shape to make a good fit with Dot1L could unveil opportunities to find new treatments targeting that connection.
Scientists Piece Together How the DNA Copying Machine Works
It happens trillions of times throughout the human body: Tiny molecular machines copy DNA inside a cell and make two, hopefully exact, copies of 6 billion pieces of DNA without making a typo.
That precision is remarkable, along with the fact that it’s happening on such a small scale.”
James Berger, Ph.D., Director of the Institute for Basic Biomedical Sciences and Professor of Biophysics and Biophysical Chemistry at the Johns Hopkins University School of Medicine
Scientists use the term “replisome” to refer to the molecular machine that copies DNA. The replisome is a collection of proteins and enzymes that link together to form the DNA copying machine. “We understand how different pieces of the replisome work, but we don’t understand how they work together,” says Berger, whose lab specializes in piecing together how DNA copies itself.
The replisome, says Berger, is like a self-feeding photocopier, reeling in one piece of DNA and spitting out two copies. The motor that runs the copy machine is called a helicase. It unpairs and unwinds the double strands of DNA so the copying machinery can access and copy the molecular information stored in genetic code. Like many car motors, helicases are powered by six cylinders, or “rings,” that encircle and move along DNA threads.
Scientists Piece Together How the DNA Copying Machine Works
Animated version of a loading enzyme opening its target helicase ring. DnaC is shown in orange and the helicase ring in dark/light blue. A single DNA strand appears in red at the end of the animation, and causes the helicase ring to snap shut. Credit: James Berger.
Berger’s team looked at bacteria to figure out how an enzyme called DnaC loads one of the helicase rings onto DNA. In a report published in February in Molecular Cell, the scientists found that DnaC binds to the helicase and, using one of its six arms, tickles the ring to crack it open and attach the ring to the DNA strand. Then, DnaC shuts off.
Berger’s lab is continuing to study how DnaC is ejected from the copy machine complex, how the helicase motor bolts onto the copying complex and how the helicase moves along DNA. The findings will help pave the way toward targeting the helicase for anti-bacterial therapies and will provide insights into how genetic disease arises when a mutated helicase malfunctions.
Source:
Johns Hopkins Medicine
Journal reference:
Wolberger, C. et al. (2019) Mechanism of Cross-talk between H2B Ubiquitination and H3 Methylation by Dot1L. Cell. doi.org/10.1016/j.cell.2019.02.002.