The virus responsible for chickenpox and shingles employs a powerful strategy of immune evasion, inhibiting the ability of natural killer cells to destroy infected cells and produce molecules that help control viral infection, according to a new study.
The research which was conducted by Allison Abendroth of the Discipline of Infectious Diseases and Immunology in the Charles Perkins Centre at the University of Sydney, and colleagues, was published on June 13 in the open-access journal PLOS Pathogens.
As the authors noted, future studies that elucidate the underlying molecular mechanisms may allow exploitation of this knowledge in therapeutic settings in which aberrant natural killer cell activation can cause immunopathology, such as in certain autoimmune diseases, graft-versus-host-disease, and transplant rejection.
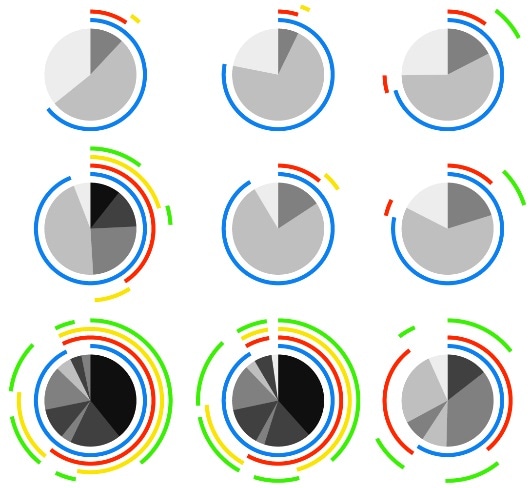
Polyfunctional analysis of VZV cultured NK cells.
PBMCs were mock cultured, exposed to VZV, or VZV infected for 2 days and stimulated as specified for flow cytometry analysis of NK cells (viable CD3–CD56+ cells).
Credit: Campbell, et al. (2019)
Natural killer cells are white blood cells that help control viral infection by killing infected cells and secreting molecules called pro-inflammatory cytokines to activate and direct the immune response. In retaliation, viruses such as varicella zoster virus (VZV), which causes chickenpox and shingles, dampen the immune system to establish infection in humans.
VZV can infect human natural killer cells, but it was not known how, or if, VZV could directly affect natural killer cell function. To address this gap in knowledge, Abendroth and her colleagues cultured human peripheral blood mononuclear cells with VZV-infected cells, and then assessed the functional capacity of natural killer cells.
The researchers identified a previously unreported strategy employed by VZV to powerfully inhibit natural killer cell function, hindering the ability of natural killer cells to destroy infected cells and produce cytokines. This powerful impairment of function is dependent on direct contact between natural killer cells and VZV-infected cells.
In this way, VZV paralyzes natural killer cells from functionally responding to target cells. The closely related virus, herpes simplex virus type 1, is also capable of efficiently infecting natural killer cells and rendering natural killer cells unresponsive to target cell stimulation.
According to the authors, these findings indicate for the first time that two human alphaherpesviruses directly target multiple anti-viral functions of natural killer cells for immune evasion.
We now have a clearer understanding as to how herpesviruses are able to manipulate the host immune response.”
Source:
Journal reference:
Campbell, T.M. et al. (2019) Functional paralysis of human natural killer cells by alphaherpesviruses. PLOS Pathogens. doi.org/10.1371/journal.ppat.1007784.