This agent causes a naturally occurring fluorescent molecule called protoporphyrin IX (PpIX) to accumulate inside the tumor cells selectively. PpIX is subsequently released from the cell inside little packages called extracellular vesicles (EVs). When blood samples from these patients are viewed under blue light microscopy, the PpIX-positive EVs fluoresce pink, allowing preoperative diagnosis of the glioma. This finding could also possibly allow the tumors to be monitored for recurrence following surgery.
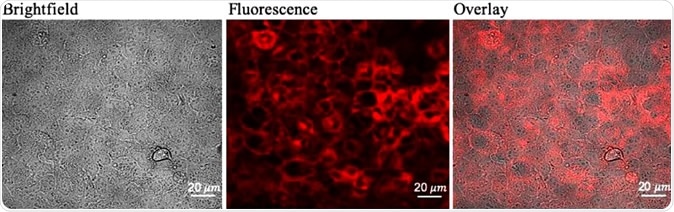
Determining optimal 5-ALA dose for maximum fluorescence and viability of glioma cells (a) Confocal images of PpIX fluorescence in Gli36 cells treated with 0.8 mM 5-ALA dose for 24 h. Brightfield image (left), fluorescence image (center), overlay image (right).
What is already known
The use of the imaging agent 5-ALA is approved by the US Food and Drug Administration for brain tumor surgery. The uptake of 5-ALA makes the tumor fluoresce bright pink under blue light microscopy, allowing the surgeon to distinguish tumorous tissue from normal non-fluorescent tissue. Fluorescent-guided surgery is associated with better tumor removal and a longer progression-free survival compared to resection under normal conditions. Removing the whole of the tumor surgically is the treatment of choice in glioma patients, so being able to visualize the tumor makes it easier to see if any tumor remains.
The principle
The fluorescence of the tumor cells following 5-ALA administration is due to the accumulation of a heme precursor, a naturally occurring fluorescent molecule, called tumor-specific protoporphyrin IX (PpIX). In the presence of 5-ALA, PpIX builds up inside the glioma cells to very high concentrations. For instance, the blood-brain barrier in gliomas is much weaker than normal. Several brain chemicals like GABA, ABCG2, pepT1 and pepT2 transporters also actively carry 5-ALA into the glioma cells. The high level of 5-ALA inside the tumor cell causes an altered pattern of enzyme activity, which results in the accumulation of PpIX. When the cell containing PpIX is exposed to blue light, it fluoresces a bright pink.
The study
All cells release subcellular enclosed components called extracellular vesicles (EVs). Glioma cells in the brain do this too, but these EVs also contain PpIX which fluoresces, indicating their origin. The present study aimed to detect and examine EVs in peripheral blood after a drink of 5-ALA, to see if this will provide a minimally invasive way to test for the presence and volume of a malignant glioma.
Glioma cells
The current research shows that glioma cells in the brain exposed to 5-ALA express almost 250 times as many EVs containing PpIX as glioma cells without this exposure. These EVs will show up pink on fluorescence, just like 5-ALA-exposed gliomas do. This could then be a quick way to detect primary or recurrent brain tumors.
Glioma-carrying mice
In the second step, the scientists observed that following the administration of 5-ALA to mice with gliomas and to healthy mice, the number of PpIX-containing EVs in peripheral blood is much higher in the first group. When blood samples taken from tumorous mice before and after 5-ALA administration are compared, the 5-ALA causes a marked increase in the level of EVs containing PpIX. In other words, giving mice 5-ALA to drink increases the PpIX-positive EV number only in mice with glioma EVs. In the words of Leonora Balaj, a researcher in the current project, “If there is no tumor, then there are no pink EVs in the blood, making them highly specific to the presence of the brain tumor.”
Glioma patients
The researchers then gave patients 5-ALA before they had brain surgery for cancer under fluorescent guidance. A blood sample was taken before and after the drink. They found that tumors which showed intense fluorescence during the surgery were associated with a much higher number of fluorescent EVs in the preoperative post-5-ALA blood sample. Moreover, the number of pink EVs in the patient’s blood was directly related to the size of the tumor.
Future implications
These findings could help fulfil the clinical need to diagnose the brain cancer without actually having to open up the skull. Neurosurgeon Bob Carter comments, “Characterizing circulating tumor-specific fluorescent EVs provides a window into the primary tumor's presence and status.”
The scientists plan to continue administering 5-ALA to preoperative glioma resection patients to validate this test as a marker for primary glioma. They also feel that the same test might be useful in diagnosing a recurrent tumor as well. After brain surgery, intense inflammation, the presence of dead and dying tissue around the site of tumor resection, and scar formation, all serve to confuse the images acquired by various technologies. This could in fact lead doctors to think they are watching a tumor recur or grow – a phenomenon called “pseudoprogression.”
Lead author Paula Jones says, “We plan in the future to test the use of 5-ALA in patients who come back with a suspected recurrence to be able to determine if it can also distinguish recurrent tumors versus other inflammatory conditions.”
Journal reference:
Characterization of plasma-derived protoporphyrin-IX-positive extracellular vesicles following 5-ALA use in patients with malignant glioma. Pamela S. Jones, Anudeep Yekula, Elizabeth Lansbury, Julia L. Small, Caroline Ayinon, Scott Mordecai, Fred H. Hochberg, John Tigges, Bethany Delcuze, Alain Charest, Ionita Ghiran, Leonora Balaj, & Bob S. Carter. EBioMedicine. October 15, 2019. https://doi.org/10.1016/j.ebiom.2019.09.025. https://www.ebiomedicine.com/article/S2352-3964(19)30629-2/fulltext