Enzymes are biological proteins that catalyze a range of chemical reactions under the mild conditions of a living organism. That is, they can be activated at normal temperatures and pressures. In contrast, most industrial catalysts act only at extreme temperature and pressure, as well as poisonous solvents or metals.
The problem with using only enzymes for catalysis is that they aren’t available for all chemical reactions. The modern reaction is to make it if it isn’t there. One way to do this is to change an already existing enzyme so that it works. Another is to create a new enzyme.
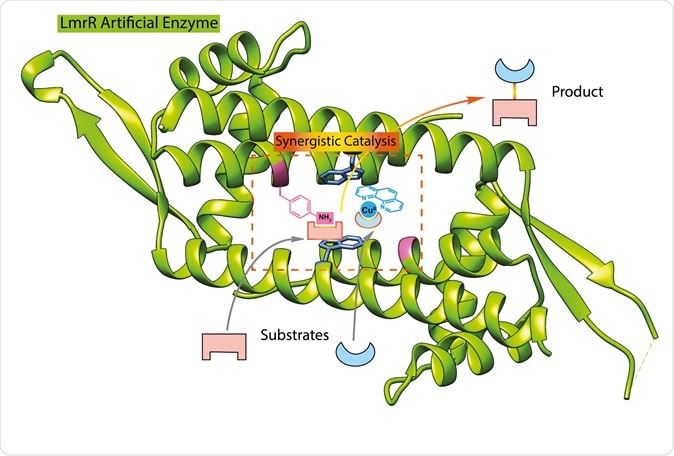
This illustration shows the structure of the LmrR protein (green), with the added catalytic groups binding to substrate. | Illustration Reuben Leveson-Gower
The catalytic group
The issue with the first approach, according to the researchers, is that it involves reducing enzyme capability. One of them, Gerard Roelfes, says, “Natural enzymes evolved to catalyse specific reactions. Adapting requires a kind of devolving of the enzyme. That is why we pioneered the creation of new, unnatural enzymes.”
Enzymes often owe their high catalytic activity to the way the catalytic side chains are placed within a protein-binding pocket. The scientists therefore tried to play around with the binding pocket, via genetically induced variations in the positioning of the unnatural amino acid and therefore of its side chain. They found that several mutations could increase the enzyme’s turnover by 100 times.
The first enzyme he synthesized was one which can form unnatural hydrazones. It is based on a biological molecule called LmrR, which is basically a bacterial transcription factor, without enzyme activity. It is shaped like a doughnut with a central large pore that serves as a base to link flat hydrophobic molecules.
In 2018, this protein was modified by the insertion of the unnatural amino acid p-aminophenylalanine, at which point it became an enzyme. This amino acid contains an aniline side chain which is responsible for catalysis.
The sidechain
The sidechain attaches itself to the LmrR protein, by what are called supramolecular bonds – that is, bonds formed as a result of the enzyme’s interactions with the transcription factor. The researchers explain, “Its position is determined by the interaction with the protein. From this position, we determined where the p-aminophenylalanine should be inserted into the protein to create an active site. From my experience in organic chemistry, I knew the potential utility of this aniline side chain for catalysis and envisioned that it would be possible to combine it with copper catalysis.”
Using this idea, the researchers built a new enzyme which had highly selective activity for the Michael addition.
This time around, the researchers adapted this same protein, but then added in the copper complex to the bacterial protein, before inserting the p-aminophenylalanine. The latter’s aniline chain combined with copper to act as a powerful catalyst for this reaction. Copper is a well-known transitional metal catalyst due to the presence of variable oxidation states, allowing them to accept and donate electrons with ease.
Both of these sites cooperate to catalyze the reaction partners for the Michael addition reaction, a common organic reaction that is used to create bonds between carbon atoms. However, just putting both of them into a molecule doesn’t work, because they counteract each other at too close distances. Says Roelfes, “They both have to be in the right position to efficiently and selectively catalyse this reaction.”
Admittedly, the enzyme is a relatively slow one. Roelfes explains, “The enzyme is not yet fast enough for practical use but, with standard techniques such as directed evolution, this could be improved.”
The concept
The important thing they achieved is that they showed a proof of concept. Two abiological or unnatural catalysts were brought together for the first time to build a completely new enzyme. They feel that this could serve as a launchpad for the synthesis of many different types of new enzymes.
The importance of the technique is that it was designed to be used by ordinary chemists, though it involves molecular biology methods for the incorporation of the unnatural amino acids. This has made it possible for the team to continue to produce new synthetic enzymes.
The major use of unnatural amino acids in enzyme synthesis so far was to try to elucidate the mechanisms behind their catalytic properties, and to understand how the enzyme function was linked to its molecular structure. Roelfes and his researchers have expanded this to include the use of novel amino acids via genetic code, within enzymes. He says, “To me, the generation of new-to-nature enzymes is the most exciting option.” The addition of unnatural amino acids to a protein to create a new enzyme could be adopted to make many more, covering the complete range of standard chemical reactions. This would change the face of chemistry reactions, making them more environmentally friendly and also reducing the energy inputs.
Journal reference:
Drienovská, I., Roelfes, G. Expanding the enzyme universe with genetically encoded unnatural amino acids. Nat Catal (2020). https://doi.org/10.1038/s41929-019-0410-8