Celltrion Group today announced key milestones in its efforts to fight the 2019 novel coronavirus (COVID-19) pandemic. As part of its emergency preparedness plan to address the outbreak, which has infected over 200,000 people worldwide, Celltrion has successfully completed the first step of developing an antiviral treatment to fight COVID-19 and aims to launch a rapid self-testing diagnostic kit that could provide results within 15-20 minutes.
Celltrion has been selected as a preferred developer for a monoclonal antibody project to treat and prevent COVID-19 by the Korea Centers for Disease Control (KCDC). Korea was one of the first countries to be affected by the global pandemic. Celltrion has identified the library of antibodies sourced from the blood of recovered patients in Korea, which are thought to be involved in neutralizing the virus and may contribute to recovery from COVID-19. These antibodies are undergoing further screening processes to identify those that are most effective in neutralizing the virus causing COVID-19. Once identified these will form the basis of the antiviral treatment to be tested through pre-clinical and clinical trials around the world in the third quarter of 2020.
Furthermore, Celltrion plans to develop a ‘super antibody’ that can attach and neutralize all kinds of coronavirus related strains, such as those causing COVID-19 and SARS, enabling further protection against unforeseen or unexpected mutations. Celltrion hopes the ‘super antibody’ candidate could contribute towards preparedness for potential future pandemic situations.
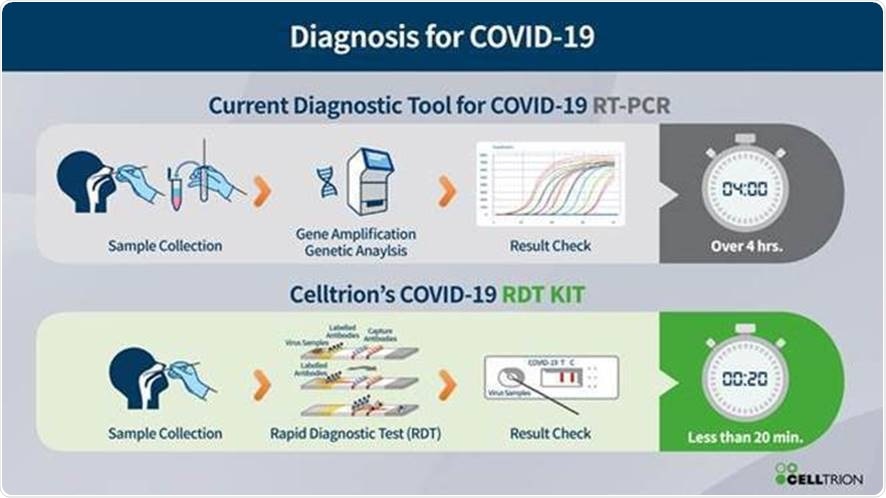
Alongside therapeutic antibody development, Celltrion aims to launch a rapid self-testing diagnostic kit in the summer of this year. The kit will focus on the gene that encodes the surface spike (S) protein; an essential glycoprotein for viral entry into human host cells. The kit is designed to show results within 15-20 minutes, with optimized sensitivity, specificity and improved accuracy features. Once it has gained a CE mark, the rapid self-testing diagnostic kit will become available throughout Europe through Celltrion Healthcare. Celltrion plans to apply for device authorization from the FDA in the US and other regulatory authorities after acquiring relevant data.
Our COVID-19 antiviral treatment is designed to train the immune system to make antibodies that recognize and block the spike protein that the virus uses to enter human cells. Antibodies that bond to the COVID-19 antigen will also be used to develop a diagnostic kit. We implemented an emergency preparedness plan at the very start of the COVID-19 outbreak leveraging our unique antibody discovery, development and manufacturing technologies. We remain dedicated to supporting healthcare systems across the world and innovating to support patients throughout this pandemic.”
Ki-Sung Kwon, Head of R&D Division at Celltrion