The study was based upon the use of cryo-electron microscopy to acquire images of the transport protein. The tuberculosis bacterium is a complete organism in that it has all the necessary genetic machinery to produce cobalamin in its cell. However, it has to import the vitamin from outside for a successful cell division.
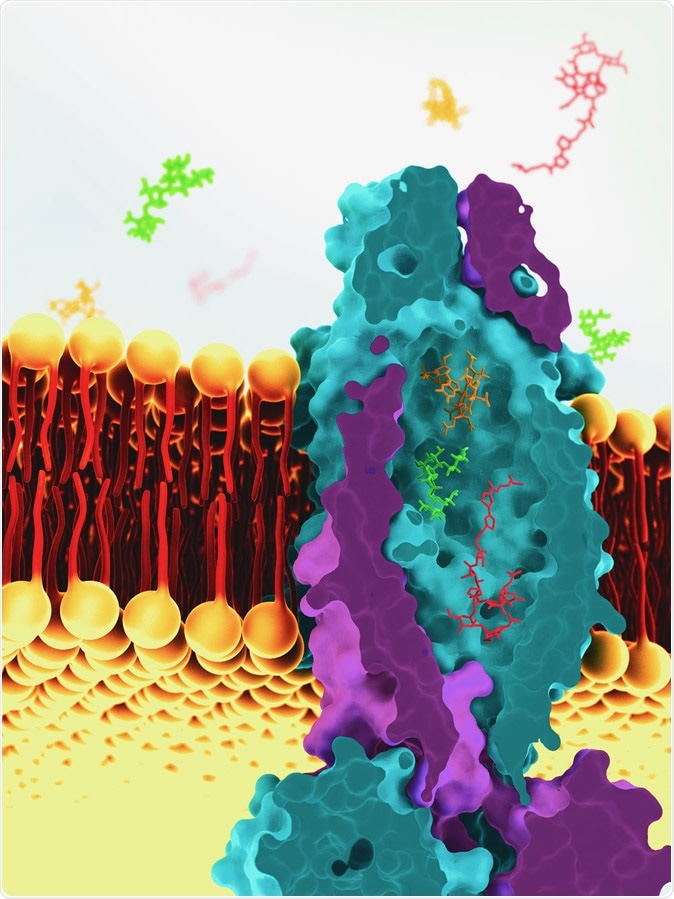
This is a reconstruction of the vitamin B12 transporter from Mycobacterium tuberculosis, based on cryoEM images. The transport molecule sits across the cell membrane of tuberculosis bacteria and helps to ferry molecules into the cell. Image Credit: Greg Stewart / SLAC National Accelerator Laboratory
ABC transporters
To accomplish this process, it uses a vitamin B12 transporter protein, one of a large family of ATP-binding cassette (ABC) transport proteins. These proteins make use of the chemical energy stored within ATP molecules to carry the substrate molecules across the cell membrane. These transporters are also involved in the transport of peptides like bleomycin, which inhibits microbial growth. This is an oddity, according to researcher Dirk Slotboom, who says two very diverse molecules are seldom carried by the same transporter.
This motivated the current study to examine the structure of this unique protein. Says Slotboom, "'This was a long process, but we finally cracked it using cryo-electron microscopy." And the results were amazing: the protein contained what can only be described as massive, a large cavity filled with water extending over the entire width of the cell membrane, with a capacity of 7,700 cubic Angstrom – as much as seven cobalamin molecules, according to the researcher.
How it works
The working of the transporter appears to be simplicity itself: just empty itself out, with all that is in the water. Hence the sluice analogy. Slotboom goes on to explain: "You let the water in and everything that is in it." This is probably how it can transport both the vitamin and the antibiotic peptide, despite their very different structures.
The nonselective transport capability also has its drawbacks, the most obvious of which is that it is quite inefficient. However, this is not an issue in this situation, where the bacillus needs to take up only a limited number of cobalamine molecules to complete its reproductive cycle lasting about 24 hours.
A unique transport protein
The researchers are taken aback by the difference between this transporter and any other known conventional transport protein. They comment, "It changes the way that we look at the physiology of bacteria. There are strong indications that other bacterial species have a similar system, which means that they pick up random molecules from their environment."
On the other hand, the investigators are intrigued by the possibility that human cells might also have a very similar mechanism to transport substances like cobalamin. This vitamin first binds a gastric peptide called intrinsic factor. This peptide, from the specialized gastric parietal cells of the stomach lining, allows vitamin B12 to form a complex with it. This complex is then taken up by the epithelial cells.
The complex finally lands up in the lysosomes within the epithelial cells. Lysosomes are 'suicide bags' full of potent enzymes. Here, the intrinsic factor is broken down, and B12 released from the lysosome to enter the cells. Here it finally takes part in the metabolism of the cell. Slotboom says, "I strongly suspect that this involves a similar non-specific transporter."
The scientists also think they might be able to stimulate the activity of the cobalamin-bleomycin transport protein to help treat tuberculosis. They say, "'If we could stimulate the activity of this transporter, it might import antibiotics more efficiently, making it easier to kill these cells. We realize, though, that this may not be straightforward, as the bacterium uses effective strategies to keep antibiotics out."
To accomplish this, scientists are analyzing the working of the transporter next. The current hypothesis is that "Inside the cell, the sluice is emptied by binding and hydrolyzing ATP. But we do not know how it opens on the outside, to let new molecules in."
The dimeric transporter is composed of two halves. These seem to project to the outside of the cell membrane, and it is possible that they open in some way, like a gate, to allow fresh cargo to enter the cell. The scientists want to see if they can somehow stimulate this opening or loosening process so as to increase the entry of antibiotics.
Journal reference:
Reference: S. Rempel, C. Gati, M. Nijland, C. Thangaratnarajah, A. Karyolaimos, J. W. de Gier, A. Guskov & D. J. Slotboom: A mycobacterial ABC transporter mediates the uptake of hydrophilic compounds. Nature, 26 March 2020, http://dx.doi.org/10.1038/s41586-020-2072-8