My academic career was highly gravitated towards researching a novel, yet highly pragmatic, 3D optical microscopic imaging modalities for biomedical applications, with a focus on stain-free 3D imaging of biological cells in vitro.
Of the various application domains for these technologies, IVF is a very prominent one where one would like to obtain “much more” information about the observed cells, whilst minimizing the damage to these cells.
As a comparison, in typical pathology, you are free to use staining chemicals on the biological sample, as following the analysis, the sample is either discarded or archived, but is never used back in the human body.
Image Credit: Maxx-Studio/Shutterstock.com
What is IVF?
In its narrower (and more accurate) definition, conventional IVF (in vitro fertilization) is a procedure where the female egg is placed inside a petri dish together with a large population of sperm cells and some supportive medium, and fertilization of the egg by one of the sperm cells is anticipated to take place.
The more advanced and prevalent type of IVF is known as ICSI (intracytoplasmic sperm injection), as some studies have shown a greater chance of fertilization when directly selecting and injecting a single sperm cell into the egg. These procedures relate to the general domain of ART (artificial reproduction technologies).
How did you develop a safe and accurate 3D imaging method to monitor sperm cell movement and quality?
We have developed clinical holographic setups that can visualize individual biological cells without staining with great contrast, and obtain much more information than possible with regular staining (which is not allowed in IVF or ICSI).
Holography uses the optical interference of a sample beam with a reference beam to record the delay of light passing through the sample, and thus it yields label-free quantitative contrast in the image.
This way, we record the full sample wavefront containing the optical thickness map, or the OPD (optical path delay) map of the cell, so that at each point on this map, OPD is equal to the integral of the refractive index values across the cell thickness.
We used a very weak light beam to avoid damaging the genetic material.
The figure below presents images of sperm cells acquired in my lab, which demonstrates the differences between the qualitative information provided by bright field microscopy (BFM), even if using labeling or staining to enhance contrast, differential interference contrast (DIC) microscopy, which is one of the commonly used qualitative phase imaging techniques, and holography, or interferometric phase microscopy (IPM), which allows quantitative measurements of the optical thickness of the cell on all of its points.
The values on the topographic OPD map are proportional to the dry mass surface density of the imaged cell, which is an example of a cellular parameter that has not been available to clinicians so far.
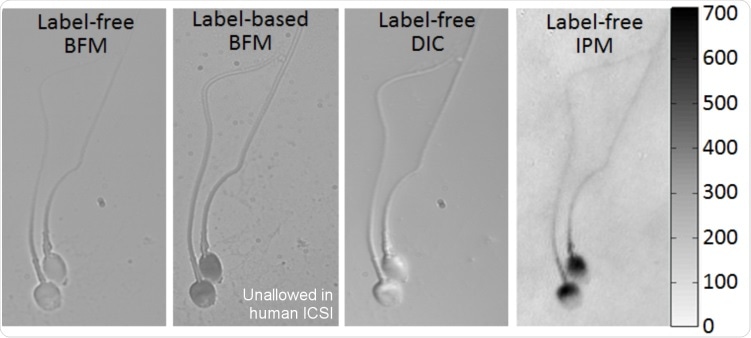
Imaging of the same sperm cells with typical qualitative microscopy methods and with quantitative IPM (holography), providing topographic OPD map, as obtained in my group. The color bar on the right represents OPD values in nm, for the holographic image. BFM = Bright field microscopy. DIC = Differential interference contrast, IPM = Interferometric phase microscopy (holography).
Holography, in general, is based on a mature technology for wavefront sensing. However, until recently it could not be implemented in clinics due to its bulkiness, non-portability, and the requirement for specific optical skills to align and use it.
In the last few years, we have made significant efforts and succeeded to make these wavefront sensors affordable for direct clinical use.
We use compact and portable modules that can be connected to existing lab microscopes and provide holographic data to the same, or even better quality, compared to these provided by the much bulkier and expensive setups.
Indeed, using these setups, we have shown that clinic-ready holographic setups can image sperm cells with excellent contrast without staining, possessing the potential for detecting DNA fragmentation in sperm cells without staining, as well as virtually staining them, meaning showing the cells as they were chemically stained (recent PNAS paper).
Holography provides just a topographic OPD map. It does not have an intracellular sectioning capability and it cannot provide the x–y–z 3D image.
To allow visualization of the full 3D image, interferometric tomography is used, where many holographic projections from multiple angles are collected and processed to generate the 3D refractive index map.
To allow the collection of holographic projections for multiple viewing angles, there are two approaches: rotating the entire sample or scanning the illumination.
However, none of these methods can cope with the problem of obtaining high-resolution 3D label-free imaging of ultra-rapid dynamic cells, like sperm cells swimming freely since rotating the sample or the illumination takes time.
In our recent Science Advances paper, we presented the first high-resolution interferometric tomography for 3D acquisition of the entire sperm cell (head with organelles and tail) during free swim, and without cell staining.
We achieved both the 3D refractive-index profile of the sperm head, revealing its fine internal organelles and time-varying orientation and the detailed 4D (space and time) localization of the thin, highly dynamic tail of the sperm cell.
The sperm head tomography is based on the fact that the sperm rotates its head naturally during free swim, so it gives us a "free" possibility to record its holographic projections. This method has great potential for both biological assays and clinical use of intact sperm cells since it provides dynamic 3D (or 4D) imaging.
See the figures below.
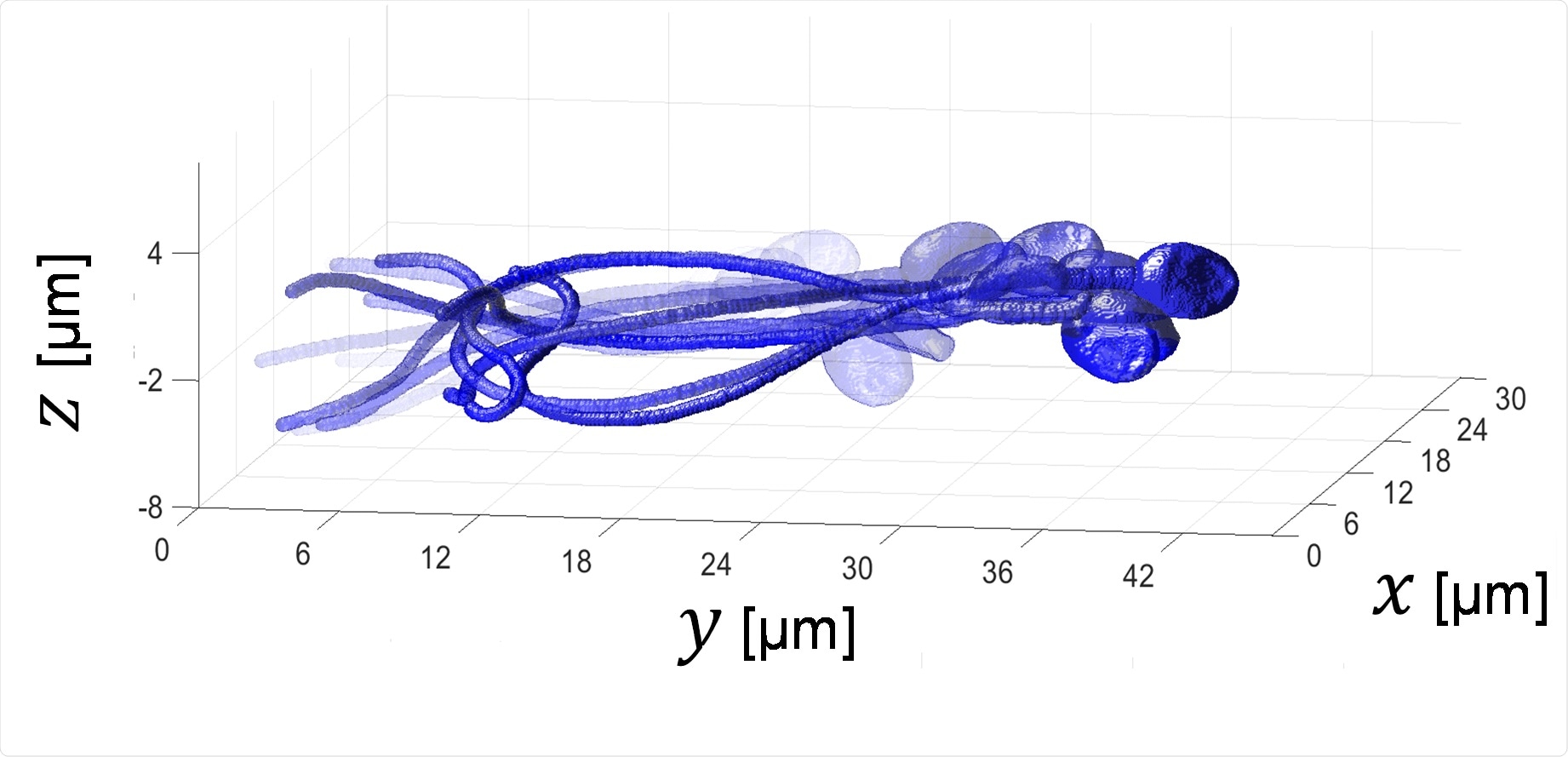
4D sperm image: 3D acquisition of a sperm cell during free swim without staining.
Acquisition rate: 2000 frames of second, Duration: half a second.
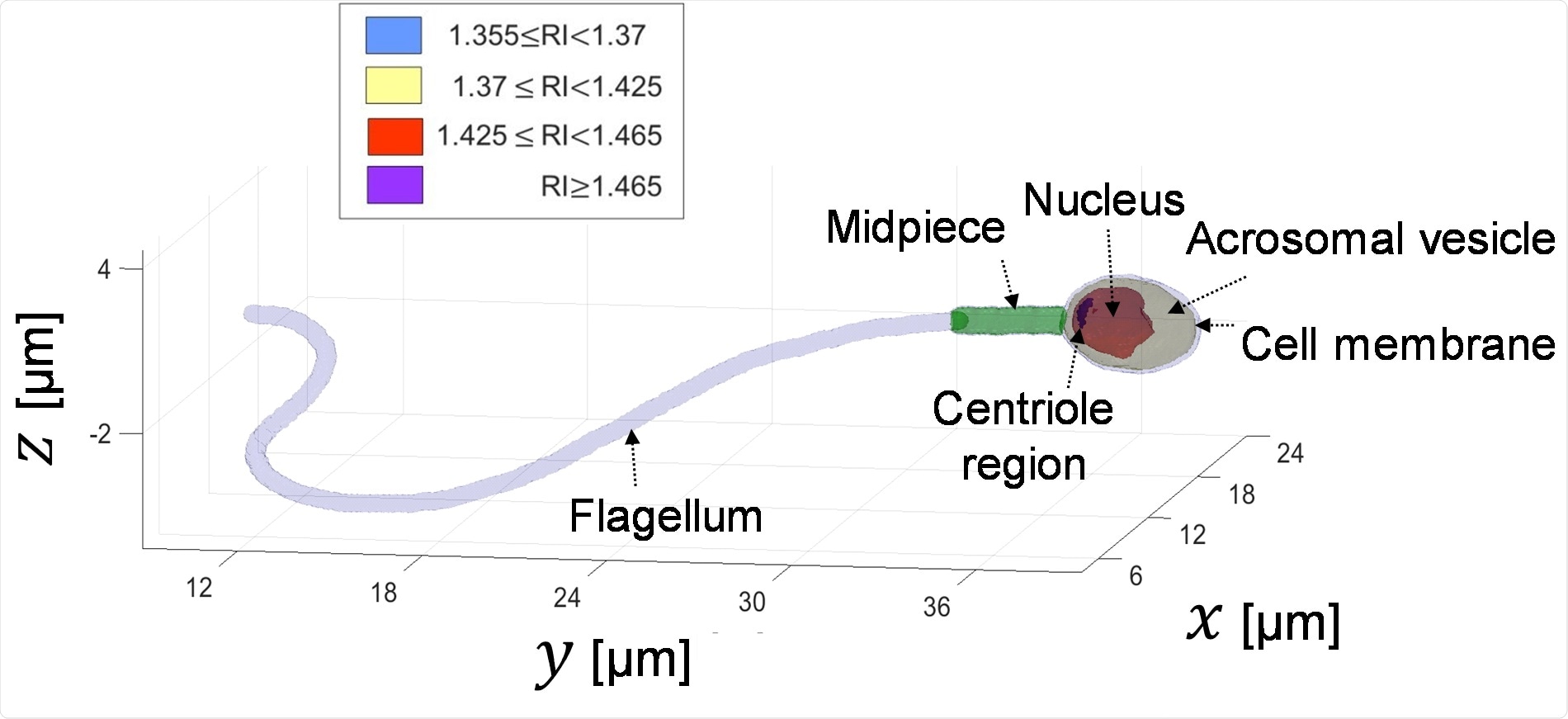
Visualization of the inner organelles of the sperm cell, which can be discriminated by the values of their refractive index (RI). This is done during the sperm swim and without staining it.
What benefits does your new imaging method have over other imaging techniques previously used in IVF?
Regarding our regular holographic techniques, the WHO (World Health Organization) and the clinical and scientific community of reproductive medicine have characterized the internal structure of “good” sperm cells, rather accurately; yet to be able to assess whether sperm cells comply with these criteria, one would need to chemically stain the cells, and by that, render them unsuitable for use in IVF.
Our holographic technique allows the embryologist to have all of the information that is required for applying the WHO criteria, as well as much more insightful information about the individual sperm cells being observed, such as DNA fragmentation level.
Now we are even better than this, since our tomographic approach provides full acquisition of the sperm 3D dynamics (and not just one holographic projection). The analysis can be either done manually by the embryologist or automatically, by the computer.
Since the 4D images of the sperm cells, which are chosen fertilization, are now recorded (in contrast to the common practice today), and they contain many new parameters, such as the sperm organelle volumes and its full 3D dynamics, a database of sperm cells can be built, analyzed by deep learning, and then be used to investigate the couple success/failure reasons, which establishes a new personalized medicine tool.
Why is it important that the imaging test is safe for use on sperm cells?
There are very strict regulatory guidelines on safety to gametes (i.e. eggs and sperm cells) that are intended to be used to create in-vitro embryos, as clearly, these would hopefully turn into babies.
Why is the staining of sperm cells not allowed in IVF?
There are different types of stains, yet, staining might need to “kill” the sperm cell, by breaking its outer membrane to permeate (i.e. penetrate) it with the particular labeling agent and internally bind to the target molecule, which may be a protein in the nucleus, the acrosome, the cytoplasm, etc.
Furthermore, human IVF procedures typically do not allow using even fluorescent dyes for live sperm cells due to the risk of damaging the sperm genetic material.
Why is the quality of the sperm so important in IVF treatments?
Better sperm selection will result in better pregnancy rates and outcomes. Clinical outcomes stand at one live birth out of approximately six IVF cycles.
Typically, all eggs are fertilized by sperm cells in an average IVF treatment because of their small number (around ten eggs), but individual sperm selection out of millions is in the heart of an average IVF treatment and have a medical, financial, emotional, social, and career-wise impact on couples trying to become parents. Each sperm cell results in a different person if the pregnancy is successful.
The very individual sperm that would eventually fertilize the egg is believed to have just as important role in determining the fate of pregnancy, as this of the egg. Typical embryo culture of either 3 or 5 days, would only allow a partial glimpse of the comprehensive “quality” of the embryo and its chances to yield live birth.
There are, indeed, relatively new techniques that are being employed as a replacement for amniocentesis, which is typically termed PGD or PGS, yet they would mostly be adequate to try and identify specific genetic traits, so these developments do not compete but rather complete each other.
Also, we do not want to face a situation in which all eggs in an IVF cycle are fertilized by defected sperm cells.
Image Credit: nobeastsofierce/Shutterstock.com
How will this method help in improving future IVF treatments?
We believe that just as any embryologist today would use his standard microscope to look at the way a candidate sperm cell swims or is generally shaped, in the not too distant future, we would be seeing many embryologists applying comprehensive screening of candidate sperm cells before individually injecting them into the retrieved eggs.
Further down the road, we intend to generate a comprehensive database of sperm cell stain-free 3D images, and together with embryo images and clinical outcome information, and using deep-learning methodologies, pave the road for next-generation big-data AI-based sperm selection.
Do you believe that your imaging technique could help in diagnosing male fertility problems?
One out of six couples suffers from fertility problems. It is believed that 1/3 of all infertility cases are due to solely male-factor, 1/3 solely female-factors, and the remaining 1/3 are combined.
The clinical assessment whether the case is in one of these groups is usually arrived at, following a few routine clinical and lab tests performed with the couple including sperm analysis.
Our technique is based on unique and direct stain-free imaging of sperm cells with a clinical level workstation.
In our study, we sought to develop an entirely new type of imaging technology that would provide as much information as possible about the spermatozoa and enable the selection of optimal spermatozoa in fertilization treatments. As explained above, we chose holographic tomography.
Using our technique, we believe a quick, cheap, and simple test can either affirm or deny these potential explanations for infertility.
In our Fertility and Sterility paper, we have shown that we can do as good as the World Health Organization (WHO) protocol for stain cells, but without staining. And this was just by using a single holographic projection.
I have co-founded, together with the CEO Alon Shalev, a company named QART Medical, that is expected to bring this technology to the clinics within the next 2 years. In the company, we built several clinical sperm imaging machines, and are expected to start clinical trials soon.
Now, we are even better since we have a very rapid 3D imaging method for the entire sperm (head and tail), without staining. So we can relate the sperm 3D dynamics to its morphology and understand the mechanisms in the woman's body of sperm selection.
What are the next steps in your research?
We have built several working prototypes in my lab for holographic imaging in clinical settings, imaged thousands of sperm cells, analyzed them, and performed various assays by experienced clinical embryologists, for our technique validation (see publications below).
We want to be able to bring this technology to clinics as soon as possible, which will allow using it for human IVF and ICSI.
Using our recent 4D imaging technique, I plan to study the sperm dynamic behaviors in various scenarios, in order to build a unified biophysical and biomechanical model that connects the sperm 3D morphology, movement, and contents.
I also plan to check the full capabilities of our new stain-free 4D imaging technique in detecting various morphological details that could not be detected so far during IVF and ICSI and quantify their clinical importance, as well as check our technique capability in measuring the DNA fragmentation level in sperm cells.
Where can readers find more information?
Company: www.qart-medical.com
Specific relevant scientific papers:
- G. Dardikman-Yoffe, S. K. Mirsky, I. Barnea, and N. T. Shaked, “High-resolution 4-D acquisition of freely swimming human sperm cells without staining,” Science Advances, Vol. 6, No. 15, eaay7619, 2020 [PDF, Supp Mat, Video 1, Video 2, Video 3, Video 4, Video 5] [Link].
- Y. N. Nygate, M. Levi, S. K. Mirsky, N. A. Turko, M. Rubin, I. Barnea, G. Dardikman-Yoffe, M. Haifler, A. Shalev, and N. T. Shaked, “Holographic virtual staining of individual biological cells,” Proceedings of the National Academy of Sciences USA (PNAS), 2020 [PDF] [Link].
- M. Haifler, P. Girshovitz, G. Band, G. Dardikman, I. Madjar, and N. T. Shaked, “Interferometric phase microscopy for label-free morphological evaluation of sperm cells,” Fertility and Sterility, Vol. 104, Issue 1, pp. 43-47, 2015 [View].
- I. Barnea, L. Karako, S. K. Mirsky, M. Levi, M. Balberg, and N. T. Shaked, “Stain-free interferometric phase microscopy correlation with DNA fragmentation stain in human spermatozoa,” Journal of Biophotonics, Vol. 11, e201800137, pp.1-10, 2018 [Link].
- P. Jacob Eravuchira, S. K. Mirsky, I. Barnea, M. Levi, M. Balberg, and N. T. Shaked, “Individual sperm selection by microfluidics integrated with interferometric phase microscopy,” Methods, Vol. 136, pp. 152-159, 2018 [Link].
- S. K. Mirsky, I. Barnea, M. Levi, H. Greenspan, and N. T. Shaked, “Automated analysis of individual sperm cells using stain-free interferometric phase microscopy and machine learning,” Cytometry Part A, Vol. 91, Issue 9, pp. 893-900, 2017 [Link].
- M. Balberg, M. Levi, K. Kalinowski, I. Barnea, S. Mirsky, and N. T. Shaked, “Localized measurements of physical parameters within human sperm cells obtained with wide-field interferometry,” Journal of Biophotonics, Vol. 10, Issue 10, 1305-1314, 2017 [Link].
About Professor Natan Shaked
Prof. Natan T. Shaked is a tenured Associate Professor and the director of The Biomedical Optical Microscopy, Nanoscopy and Interferometry (OMNI) Research Group (http://www.eng.tau.ac.il/~omni/index2.php/), a large research group which is a part of the Department of Biomedical Engineering and the Nano Center of Tel Aviv University, Tel Aviv, Israel.
Located on three lab spaces, the group performs multidisciplinary research involving optical imaging and sensing in biological systems. Until April 2011, Prof. Shaked was a Visiting Assistant Professor in the Department of Biomedical Engineering at Duke University, Durham, North Carolina, USA.
Shaked has BSc, MSc, and Ph.D. degrees in Electrical and Computer Engineering. Prof. Shaked is the coauthor of more than 80 refereed journal papers and 150 conference papers, several book chapters, patents, and an edited book.
He is chairing the SPIE Label-Free Imaging and Sensing (LBIS) annual conference in SPIE Photonics West, San Francisco, USA, and the co-founder of QART Medical Ltd (www.qart-medical.com). Prof. Shaked won many prestigious research grants including the HORIZON2020 ERC personal grant, which funded this research.