A new study by scientists from the U.S and U.K. and published on the preprint server bioRxiv* in June 2020 reports that there is no evidence of efficacy for the drug hydroxychloroquine (HCQ) against infection with SARS-CoV-2 in hamsters or macaque models. This finding does not support the current widespread prophylactic and therapeutic use of HCQ in COVID-19.
Though the COVID-19 pandemic has been spreading around the world for nearly six months, it remains a mystery in many ways. Many drugs, including HCQ, azithromycin, lopinavir/ritonavir, dexamethasone, and tocilizumab, are being used in many countries in an attempt to bring down the number of severe or critical cases.
Testing Hydroxychloroquine Effect in COVID-19
Intensive research is ongoing to discover effective drugs to arrest the mortality rate due to this infection, which is high among the elderly and those with certain underlying medical conditions. The current trial assessed the use of HCQ in hamsters and macaques exposed to the SARS-CoV-2, to prevent infection.
HCQ has been given emergency approval for use in COVID-19 patients, based on published data showing its inhibitory effect on viral replication, and some early trials using both HCQ and azithromycin in combination in this disease. However, there is currently not much data on the efficacy of HCQ in this condition in humans.
Hamster Study
The researchers first tested the drug for efficacy in cell cultures and confirmed that it inhibited viral replication. The next step was to use the drug in Syrian hamster models by injecting a single dose into the abdominal cavity at two dosages.
The first was the standard human dose for malaria prevention or treatment. The second was a much higher dose. In both cases, control hamsters were also used. The hamsters were then infected after 24 hours by a dose of virus intranasally.
Another set of two groups was first infected and then put on treatment with HCQ at the same two dosages, starting after 1 hour of infection, for three days.
For both the prevention and treatment groups, qRT-PCR was carried out on oral and rectal swabs on day 2 and day 4. The animals were euthanized on day 4, and post-mortem lung samples were also examined.
The researchers found that all animals showed the same high level of viral replication and shedding in oral swabs, with a lower load in rectal swabs. The viral loads showed a reduction in all the groups over time. The disease symptoms and signs remained consistent over the study period.
The lung specimens on autopsy showed focal but widespread areas of pneumonia. The viral loads in this tissue were very high in all four groups of treated hamsters. Similarly, the lung to bodyweight ratios was comparable in all groups.
Thus, the hamster model failed to show any significant difference between the groups following the preventive or therapeutic use of HCQ. This indicates that the drug does not inhibit either viral replication or shedding; neither does it mitigate the clinical features of the disease in this animal model.
Macaque Study
The second step of the experiment was carried out in the rhesus macaque, which is a recently developed nonhuman primate (NHP) model that develops mild to moderate features of COVID-19 after infection. The same study design was followed, with 5 macaques in the control and prophylactic groups. Both were given either vehicle or HCQ three times at intervals of a week, by oral gavage. All animals were infected on day 0, by four routes in combination – oral, intranasal, intratracheal and ocular.
Another two groups were formed with 5 members each in the control and HCQ treatment groups and treated by oral gavage from 12 hours after infection. The doses were repeated at 18, 36, 60, 84, and 108 hours after infection.
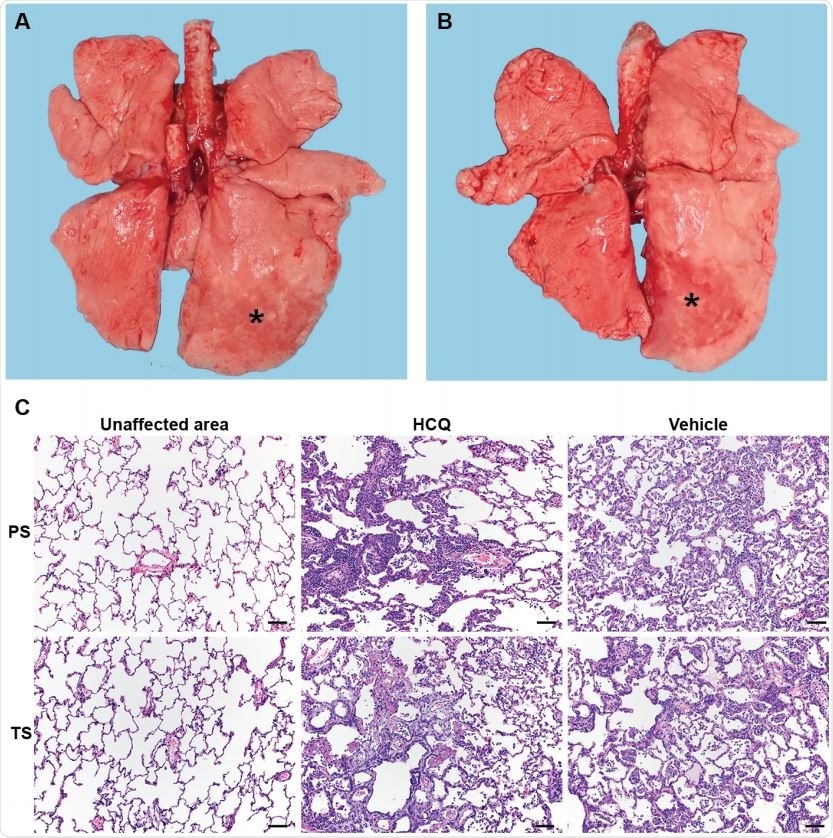
Rhesus macaque model – gross and histopathology. Macaques were infected with SARS-CoV-2 as described in the legend of Figure 2. Animals were euthanized on day 7 post368 infection for gross pathology and histopathology. (A and B) Gross pathology with consolidated lower left lung lobe and area of post-mortem-BAL in the lower right lung lobe (asterisk). (C) Hematoxylin and eosin (H&E) staining revealed multifocal, minimal to moderate, interstitial pneumonia frequently centered on terminal bronchioles. Alveolar edema and fibrin with the formation of hyaline membranes was only seen in lungs with moderate changes. Multifocal perivascular infiltrates of small numbers of lymphocytes that form perivascular cuffs. The left 3panels show areas of unaffected lung tissue. Note: PS, prophylaxis; TS, treatment.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
At 7 days from infection, all animals were sacrificed and autopsied. Until this point, twice daily monitoring was done for clinical signs and other investigations, including imaging and blood tests, as well as nasal and rectal swabs. The metabolites of the drug were also measured in the plasma and lung tissue to confirm that it was present in therapeutic concentrations.
The HCQ levels in both plasma and lung tissue were within the therapeutic range for humans. Both prevention and treatment arms showed symptoms on day 1, peaking the next day. All animals remained sick to a mild or moderate degree until day 7 when the study was ended. Lung changes in radiologic imaging were minimal in all groups.
The viral swabs were positive in all animals, with the highest viral load on day 1 and slowly decreasing up until day 7. Nasal swabs had higher loads than oropharyngeal swabs. There was no difference in viral loads between the groups. The autopsied lung specimens showed pneumonic changes in all the animals. The highest viral loads among body tissues were in the lung tissue. Thus, no HCQ effect was seen with respect to either prevention or treatment of COVID-19.
No Evidence of Hydroxychloroquine Efficacy
While convincing in vitro evidence of the inhibitory effect of HCQ on viral replication is available, in vivo proof is lacking. This finding is repeated in the current study using two accepted animal COVID-19 models. The researchers point to the inconsistency in the ongoing large-scale trial of HCQ despite the lack of evidence of efficacy. They also provide a reminder of its potential adverse effects, notably cardiac arrhythmias and liver failure.
The investigators sum up: “Independent of the safety issues associated with HCQ, the preclinical data presented here does not support HCQ and likely other 4-aminoquinolines as being either an effective prophylactic treatment to reduce SARS-CoV-2 infection or therapeutic for use in COVID-19 patients.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources