The current pandemic of COVID-19 is characterized mainly by severe acute respiratory distress of the lungs, but other organ dysfunctions are also being observed, including the heart and blood vessels. Now, a new paper published on the preprint server medRxiv* in June 2020 describes the abnormal activation of platelets in this disease and the link between this phenomenon and poor outcomes for COVID-19 patients.
The virus that causes COVID-19 is an RNA virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which binds to the angiotensin-converting enzyme 2 (ACE2) molecule on the host cell membrane via the viral spike protein. ACE2 is highly expressed on the epithelium of the nasopharyngeal airway, the type II pneumocytes of the alveoli, vascular endothelial cells, and the macrophages of the lung tissue. The binding of the virus to these sites is probably the reason for the respiratory symptoms and signs observed throughout the infection.
Cytokine Storm and Severe COVID-19
In addition to the direct injury caused by the virus, the damaged tissue releases molecules that trigger a hyperinflammatory reaction, with excessively high levels of cytokines and cell chemicals that result in further toxicity to various organs. This is called the cytokine storm and is seen in some other conditions, such as the cytokine release syndrome (CRS) seen with CAR T-cell therapy in some cancers. This probably contributes to the multi-organ damage seen in severe and critical COVID-19, involving the lungs, the heart, kidneys, and the liver.
Coagulation Abnormalities in COVID-19
Most severely ill COVID-19 patients also show clotting complications, such as microvascular thrombi, or clots in the arteries and veins. Another related condition is DIC, disseminated intravascular coagulation, in which the smaller blood vessels throughout the body form clots. Though rare in the whole population of COVID-19 patients, DIC is present in over 70% of dying patients and is, therefore, a critical element of the final events that drive the vicious cycle ending in death. Some biomarkers of this process include higher D-dimers, fibrin degradation products, prolonged prothrombin time, and activated partial thromboplastin times.
Blood tests in symptomatic COVID-19 show a deficiency of lymphocytes and white blood cells, in general. Lower platelet counts are a marker of higher mortality in hospitalized patients.
Platelets in Severe COVID-19
Some scientists feel that COVID-19 may reduce the platelet count by several mechanisms.
For instance, platelet production may be reduced, while more platelets are destroyed or consumed in intravascular clots. The current study focuses on platelet activation in COVID-19 since these cells are the main component in the formation of clots, as well as in the release of immune and inflammatory mediators.
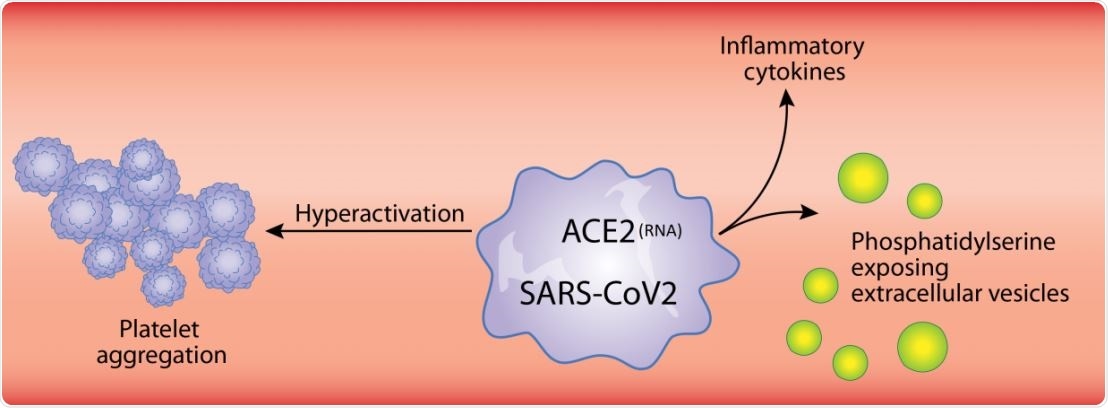
For instance, neutrophil-extracellular trap liberation (NETosis), a process in which neutrophils release extracellular DNA as a network in which cells and platelets are entangled, is a heavily platelet-dependent phenomenon. The immune and inflammatory molecules found on platelets include IL-1, Toll-like receptors, and Fc receptors for IgG FcγRIIA.
Many viruses interact with platelets and their precursor cells, megakaryocytes, leading to the enhanced expression of type I interferon genes, platelet-mediated transport, and protease activation. SARS-CoV-2 is known to enter endothelial cells, and the resulting endothelial damage may cause platelet recruitment to the infection sites. The subsequent activation and degranulation of platelets may worsen the course of the disease.
Megakaryocytes in the lung have a different immune molecule expression than those in the bone marrow. This may be a consequence of infection of these cells, and the result may be the production of platelets containing the virus within the lungs. This may, in turn, alter the transcription pattern to enhance the production of inflammatory cytokines.
The Study: Platelet Hyperactivation in COVID-19
The researchers examined over 1,500 patients with suspected COVID-19, selecting 186 patients for further analysis with RT-PCR. They looked for RNA-dependent RNA polymerase and E genes. There were 71 non-severe cases and 34 severe cases, while 81 people were negative. Non-severe patients had a mean age of 49, and severe cases 57 years, respectively.
All patients had low platelet counts, significantly lower in severe patients, but lactate dehydrogenase was higher than usual in severe patients, which is a sign of inflammation. Platelets in severe cases were found to contain viral RNA and E protein. Multiple cytokines were elevated in the blood of these patients.
Platelets were also found to produce inflammatory molecules in COVID-19 patients more strongly when stimulated by low levels of thrombin, a known trigger of inflammation, compared to healthy individuals who reacted similarly only with higher doses. Thus, platelets appear to be primed to produce certain cytokines and to release their cytokine content in COVID-19 patients.
Platelet degranulation is also seen in COVID-19, causing an inflammatory reaction. This is shown by the presence of cytokines and mediators like CD40L, PF4, and serotonin. These, in turn, can damage the blood vessel walls, and attract white cells to the spot. Platelets also release extracellular vesicles, which take part in both inflammatory and coagulation processes.
Platelet hyperactivation occurs in COVID-19, as shown by increased phosphorylation of protein kinase C δ on platelets. This enzyme is vital for the granule release from platelets, activation, and aggregation of platelets, and is significantly higher in severe patients than in non-severe cases.
Functionally, too, platelets are found to be primed so as to show an exaggerated response to lower levels of thrombin to produce a more significant amount of adherent platelets as well as to produce more inflammatory cytokines.
Implications and Therapeutic Possibilities
The study thus shows that platelet activation in COVID-19 triggers platelet activation, resulting in a higher level of systemic inflammation and intense platelet aggregation, both of which are key to the severe clinical profile in serious COVID-19.
The researchers sum up: “Hyperactivation of platelets could explain the lower platelet count and, importantly, may contribute to the thrombo-inflammatory state associated with COVID-19 infection. Therapeutic approaches to the treatment of COVID-19 may require a combination of drugs targeting platelet activities or platelet-derived molecules in addition to medications directed against other inflammatory sources.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zaid, Y. et al. (2020). Platelets Can Contain SARS-Cov-2 RNA And Are Hyperactivated in COVID-19. medRxiv preprint. doi: https://doi.org/10.1101/2020.06.23.20137596. http://medrxiv.org/cgi/content/short/2020.06.23.20137596
- Peer reviewed and published scientific report.
Zaid, Younes, Florian Puhm, Isabelle Allaeys, Abdallah Naya, Mounia Oudghiri, Loubna Khalki, Youness Limami, et al. 2020. “Platelets Can Associate with SARS-CoV-2 RNA and Are Hyperactivated in COVID-19.” Circulation Research 127 (11): 1404–18. https://doi.org/10.1161/circresaha.120.317703, https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.120.317703