The current global COVID-19 pandemic is caused by beta coronavirus severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which has infected over 22.1 million and caused 780 thousand deaths globally. One of the characteristics associated with severe and critical SARS-CoV-2 infection is the cytokine storm, a dysregulated secretion of cytokines that triggers systemic inflammation. This is a largely mysterious sequel of infection, with not too much being known about how it occurs.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The Gut and COVID-19
Prior research suggests that SARS-CoV-2 infects intestinal tissue. This has been shown by experimental infection of human intestinal organoids, as well as the high incidence of gut-related symptoms in COVID-19 patients.
Viral RNA has been found in feces for over 30 days, as well as infectious virus particles. The importance of the gut in interactions between the body and the vast world of pathogenic and symbiotic microbes is unquestioned. The gut itself harbors a body of microbes called the gut microbiota, which is vital in many body functions such as immune regulation.
IL-18, IgA, and Inflammation
An unbalanced gut microbiome is associated with many inflammatory conditions. This makes it plausible that infection with this virus could lead to abnormal inflammatory reactions that worsen the symptoms of COVID-19. The investigators in the current study looked at IL-18, a pro-inflammatory cytokine secreted by various intestinal cells such as the intestinal epithelium, immune cells, and enteric neurons. The levels of this cytokine are higher than normal in COVID-19 patients.
They also examined IgA levels, since this is the dominant mucosal immunoglobulin. It is also the most abundant immunoglobulin in humans, produced at 40–60 mg kg−1 day−1, which exceeds the total output of all other immunoglobulins put together. Moreover, the intestinal lamina propria has 8 of every 10 plasma cells in the body.
A recent paper reported the association of specific anti-SARS-CoV-2 IgA levels with COVID-19 severity. The current study extends and confirms this finding, examining how gut microbiota is altered in this condition and how it is correlated with IL-18 and specific anti-SARS-CoV-2 IgA.
Changes in Gut Microbiome and COVID-19
The study included 62 COVID-19 patients, 33 with seasonal influenza, and 40 healthy controls. All patients gave fecal and serum samples, which were sequenced. Analysis of the processed sequences showed that the gut microbiota in these COVID-19 patients was less diverse than for the patients in the other two. They were also less abundant. The researchers found that the genera Streptococcus, Clostridium, Lactobacillus, and Bifidobacterium were over-represented, and the genera Bacteroidetes, Roseburia, Faecalibacterium, Coprococcus, and Parabacteroides were fewer, relative to healthy controls.
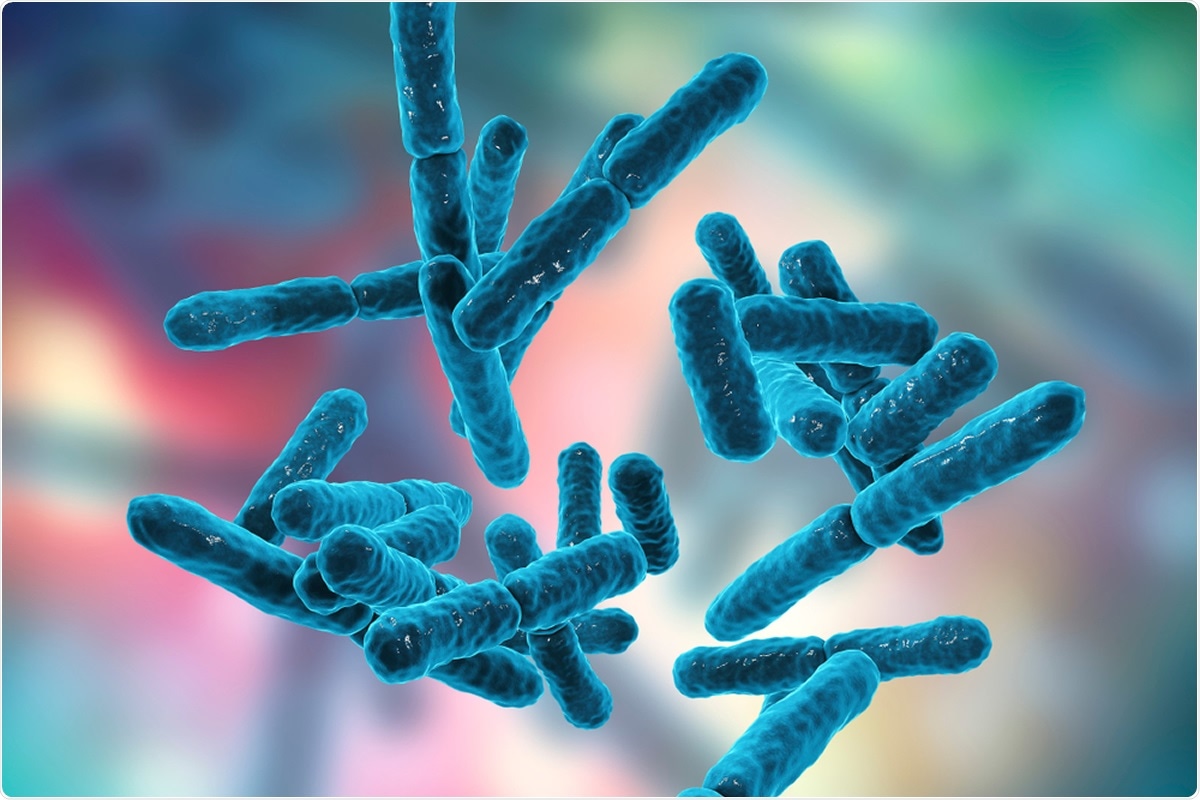
Bacteria Bifidobacterium, gram-positive anaerobic rod-shaped bacteria which are part of normal flora of human intestine are used as probiotics and in yoghurt production. 3D illustration Credit: Kateryna Kon / Shutterstock
Compared to seasonal influenza patients, members of the genera Streptococcus, Veillonella, Fusobacterium, Clostridium, Bifidobacterium, and Escherichia were increased, but the genera Parabacteroides and Sutterella were underrepresented in COVID-19 patients.
The researchers concluded, "The increased abundance of Streptococcus in COVID-19 patients was indicative of the risk of infection by opportunistic pathogenic bacteria in this group."
IgA and COVID-19
The researchers found that IgA targeting the viral spike protein was significantly elevated in COVID-19 vs. controls. It is known that viral gut infections induce IgA production. Together, these indicate that the virus causes mucosal infection. However, fecal samples failed to show significant differences in specific IgA levels, which indicates the mucosal infection probably occurred in the respiratory tract and not the gut.
IL-18 and COVID-19
Since viral infection of the gut also enhances IL-18, a pro-inflammatory cytokine, the researchers analyzed the levels of this molecule. They found that serum and fecal samples had higher IL-18 levels in COVID-19 patients compared to the other two. The levels were higher in PCR-positive cases than other COVID-19 cases, showing that the virus-induced potent inflammation in the gut.
Fecal IL-18 and Gut Microbiota
The researchers also found that fecal IL-18 levels were higher when the genera Peptostreptococcus, Fusobacterium, and Citrobacter were more abundant. This indicates, they say, that "changes in gut microbiota composition might contribute to SARS-CoV-2-induced production of inflammatory cytokines in the intestine and potentially also to the onset of a cytokine storm."
Implications
The researchers conclude that the changes in the composition of the gut microbiome in COVID-19 patients are apparent. In addition, there is a relative abundance of genera like Streptococcus, Clostridium, Lactobacillus, and Bifidobacterium, and lower relative levels of Bacteroidetes, Roseburia, Faecalibacterium, Coprococcus, and Parabacteroides in these patients. Thirdly, IL-18 levels in serum and feces are higher in COVID-19. None of these changes were seen in either seasonal influenza patients or controls.
This may suggest, they say, that "gut microbiota dysbiosis due to SARS-CoV-2 infection may contribute to disease severity, and that IL-18 might serve as an indicator of intestinal infection in COVID-19 patients." They do include an important caveat: all COVID-19 patients were being treated during the study period, which could be a confounding factor. Further studies must rule out the impact of the antibiotics and other drugs used on the gut microbiota in these patients.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources