In previous outbreaks, such as the Ebola virus disease (EVD), which ravaged through West Africa in 2014, convalescent plasma therapy has been used to treat patients infected with the deadly virus. Now, the same method is being used in many countries afflicted with the coronavirus pandemic.
Now, the U.S. Food and Drug Administration (FDA) announced that it had received an emergency use authorization (EUA) for convalescent plasma to treat patients with COVID-19.
The FDA said that based on the scientific evidence available, it had released a decision memorandum that convalescent plasma may be useful in treating COVID-19 in hospitalized patients, and the known potential benefits of use outweigh the risks.
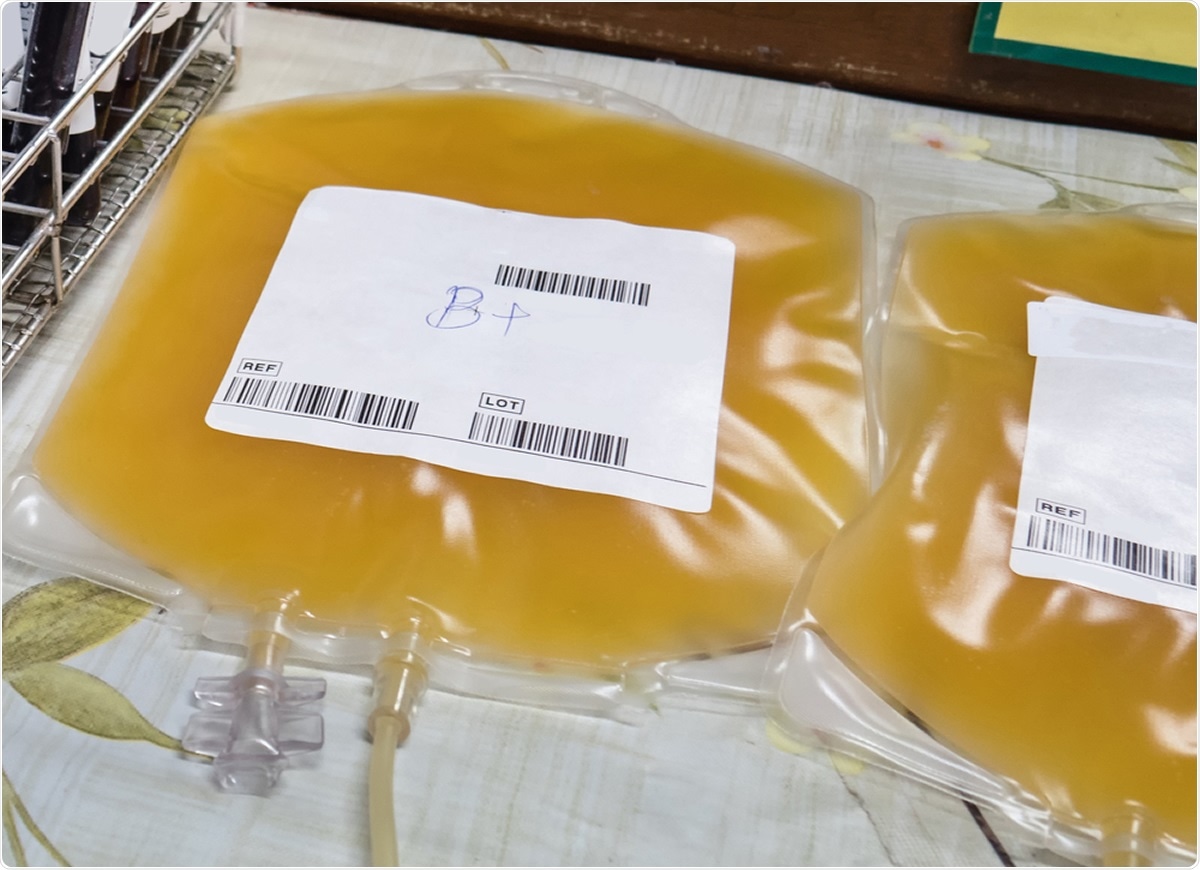
Image Credit: Room98 / Shutterstock
What is convalescent plasma therapy (CPT)?
While there is no proven treatment for coronavirus disease (COVID-19) or vaccine for the causative agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), whole blood collected from patients in the convalescent phase of infection has been used as an innovative treatment. In the new ruling, the emergency use authorization of convalescent plasma has been limited to general supportive care.
The clinical data for studies involving the infection is still scarce and limited to the hardest-hit countries early on in the pandemic, such as China, Italy, Spain, the United States, the United Kingdom, Germany, and France.
Convalescent plasma therapy focuses on passive immunity to SARS-CoV-2. There are two primary forms of immunity, active immunity, and passive immunity.
Vaccination is an example of active immunity. Injection with a foreign substance, such as viral protein, produces an active immune response from the host that results in the production of antibodies (as well as other proteins, which aid in defending the body against the viral pathogen).
Upon secondary exposure to the virus, those antibodies bind to the foreign proteins (antigens) associated with that virus and prevent viral infection or reproduction in host cells.
Passive immunity results when antibodies are “passed” from one host to another. It is a necessary form of immunity for the fetus developing in utero, and breastfed infants. Antibodies that the mother has produced against a wide variety of microbial pathogens are “passed” to the child and offer protection at their most vulnerable stage of immunological development.
The use of convalescent plasma from patients who have recovered from SARS-CoV-2 is another example of passive immunity. Antibodies from recovered patients are transfused to a new host with the goal of mediating protection by neutralizing the virus.
The convalescent plasma is the liquid part of the plasma of the blood from recovered COVID-19 patients.
Government approval
The Trump Administration announced the government’s authorization of convalescent plasma for COVID-19 patients. Trump aired his frustration at the slow pace of approval of the treatment for coronavirus treatments. He even accused health officials of playing politics regarding the issuance of an EUA for convalescent plasma.
To date, convalescent plasma has reportedly been used to treat more than 70,000 patients inflicted with COVID-19. However, just like blood, the supply of convalescent plasma is limited and must come from donors who have recovered from COVID-19.
In the United States, the number of recovered patients has topped 1.99 million people. The country has the highest number of infections and deaths, with more than 5.7 million confirmed cases and at least 176,000 people who died due to COVID-19.
“The FDA’s emergency authorization for convalescent plasma is a milestone achievement in President Trump’s efforts to save lives from COVID-19. The Trump administration recognized the potential of convalescent plasma early on. Months ago, the FDA, BARDA, and private partners began work on making this product available across the country while continuing to evaluate data through clinical trials,” Alex Azar, Health and Human Services Secretary, said.
“Our work on convalescent plasma has delivered broader access to the product than is available in any other country and reached more than 70,000 American patients so far. We are deeply grateful to Americans who have already donated and encourage individuals who have recovered from COVID-19 to consider donating convalescent plasma,” he added.
Not intended to replace clinical trials
The FDA, after reviewing data related to COVID-19 convalescent plasma, concluded that the method might be useful in lessening the severity or shortening the length of COVID-19 in some patients who are hospitalized.
However, the FDA emphasized that the EUA does not intend to replace randomized clinical trials. It is still imperative for human trials to take place, facilitating the enrollment of patients for the trials that is important to determine if the treatment is safe and effective against COVID-19.
“The FDA continues to recommend that the designs of ongoing randomized clinical trials of COVID-19 convalescent plasma and other therapeutic agents remain unaltered, as COVID-19 convalescent plasma does not yet represent a new standard of care based on the currently available evidence,” the FDA clarified.
U.S. fast-tracking candidate vaccine
The Trump administration said it considers fast-tracking the experimental coronavirus vaccine developed in the U.K., for use in the U.S.
The investigational vaccine, ChAdOx1 nCoV-19 vaccine, was developed by scientists at the University of Oxford and AstraZeneca. Meanwhile, AstraZeneca said it has not yet discussed an emergency use authorization for its vaccine with the U.S. government. Also, a Health and Human Services (HHS) spokesperson said that fast-tracking a coronavirus vaccine before the U.S. elections are “absolutely false.”