Based on structural similarities with other targets of the gamma-secretase system, researchers from Columbia University Irving Medical Center in the United States show that the SARS-CoV-2 receptor for cell entry may be affected by its proteolytic activity. Their results are currently freely available in a bioRxiv* preprint paper.
The coronavirus disease (COVID-19) health crisis, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused massive disruption worldwide with no drug or vaccine yet on sight. However, some primary research endeavors show promising results in the design of an effective and targeted therapeutic.
Angiotensin-converting enzyme 2 (ACE2) is a membrane-linked ectoenzyme that processes Angiotensin II but also mediates the entry of SARS-CoV-2 into the human cell by means of binding the viral spike glycoprotein.
More specifically, spike glycoprotein binding to ACE2 sets off membrane fusion and viral entry, but only after spike priming by transmembrane protease serine 2 (TMPRSS2), which also cleaves the ectodomain of ACE2.
ACE2 cleavage (or shedding) can additionally be fostered by the disintegrin and metallopeptidase domain 17 (ADAM17), found to compete with TMPRSS2. Accordingly, there are conflicting reports of ADAM17-mediated shedding that affect coronavirus cell entry.
.jpg)
Human ACE2 receptor, 3D illustration. Image Credit: Kateryna Kon / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The role of gamma-secretase
The gamma-secretase (γS) protein complex is comprised of a Presenilin 1/2 aspartyl protease catalytic core with regulatory (Aph-1a or Aph-1b), enhancer (PEN2), and targeting (Nicastrin) subunits. In short, this is a prototype intramembrane-cleaving protease (I-CLiP).
Dozens of putative γS targets have been identified, determined by a rather specific transmembrane conformational structure and accessibility. Nonetheless, the validation of novel targets is hampered by the lack of definite common features and ectodomain shedding demands.
Based on the structural similarity of ACE2 to known γS targets, the researchers from the Columbia University Irving Medical Center in the United States (led by Dr. Alberto Bartolomé) hypothesized that γS can regulate intramembrane cleavage of ACE2 and may impact the biology of SARS-CoV-2.
From cell cultures to advanced methods
A myriad of different methods was used to study the aforementioned hypothesis. For cell culture purposes, the researchers have used Presenilin-deficient (Psen1/2 double knockout) and control mouse embryonic fibroblasts (MEFs), as well as Nicastrin knockout and control MEFs.
Furthermore, to test the hypothesis whether ACE2ΔE and γS physically interact, the researchers performed co-immunoprecipitation of endogenous γS with C-terminally tagged ACE2 and detected the association with both Nicastrin and Presenilin1 with ACE2ΔE, but not full-length ACE2.
Finally, Western blotting, quantitative polymerase chain reaction (PCR), gel electrophoresis immunofluorescence and confocal imaging were used. Recombinant Indiana, vesicular stomatitis virus, expressing SARS-CoV-2 spike glycoprotein, has been generated as a pseudovirus.
What did this study find?
"We found that after ectodomain shedding, ACE2 is targeted for intramembrane proteolysis by γS, releasing a soluble ACE2 C-terminal fragment", say study authors. "Consistently, chemical or genetic inhibition of γS results in the accumulation of a membrane-bound fragment of ectodomain-deficient ACE2", they add.
Interestingly, this study has found ACE2ΔE accumulation even in unstimulated cells expressing ACE2 - suggesting that endogenous ectodomain shedding accompanied by γS cleavage is inherent to the normal turnover of ACE2.
Furthermore, it was shown that ACE2 undergoes TMPRSS2/ADAM17-dependent γS cleavage, which results in short-lived ACE2 C-terminal intracellular domains. Considering the latter, chemical or genetic γS inhibition prevents its generation, leading to the accumulation of a membrane-bound ACE2 lacking the ectodomain.
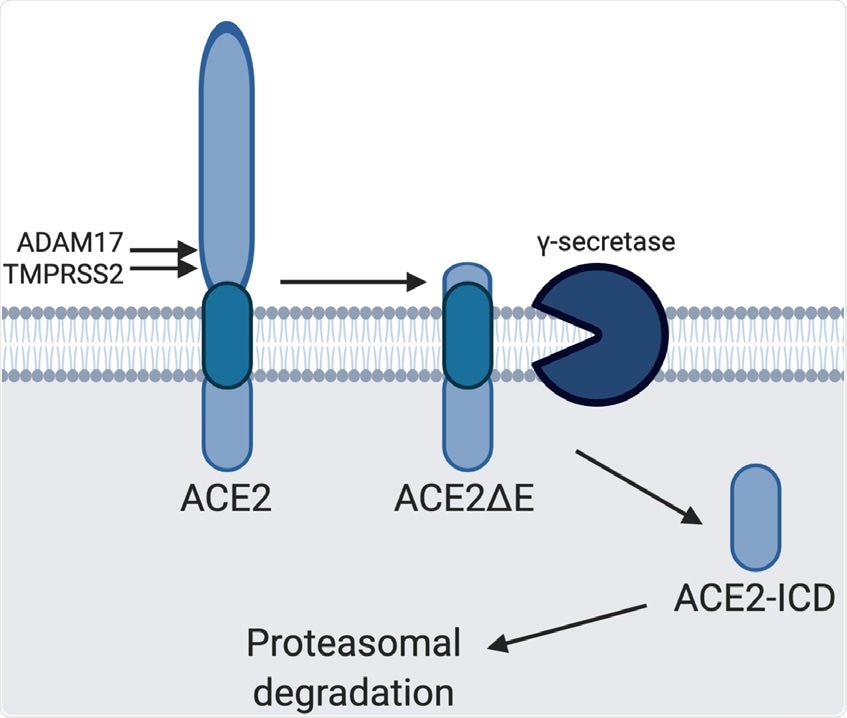
Model of ACE2 cleavage. Model showing the sequential processing of full length ACE2 by ADAM17/TMPRSS2 and γS, rendering ACE2ΔE and ACE2-ICD, respectively. ACE2-ICD is then rapidly degraded in the proteasome.
Fine-tuning for COVID-19 treatment
"In sum, our results demonstrate that ACE2 is a novel γS target, but that pharmacologic inhibition of γS does not impact SARS-CoV-2 S-protein mediated cell entry", emphasize study authors in this novel bioRxiv paper.
And albeit chemical inhibition of γS does not alter SARS-CoV-2 cell entry, these data suggest an entirely new pathway for cellular ACE2 trafficking. Such findings may have direct implications for therapeutic and other applications.
More specifically, given the optimal pharmacologic accessibility of γS, further exploration into this novel biology is warranted in order to fine-tune it against COVID-19, which is possible due to a plethora of functions ascribed to ACE2.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources